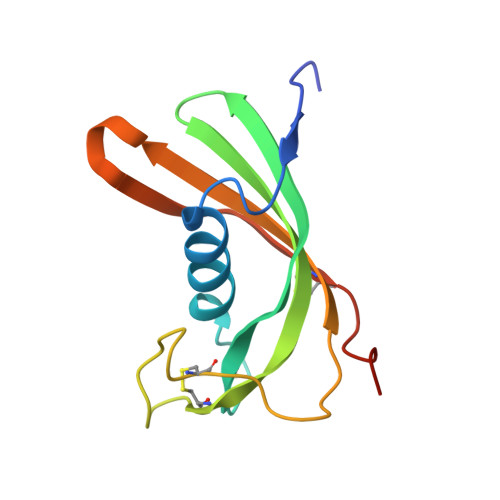
The structure and function of Iristatin, a novel immunosuppressive tick salivary cystatin.
Kotal, J., Stergiou, N., Busa, M., Chlastakova, A., Berankova, Z., Rezacova, P., Langhansova, H., Schwarz, A., Calvo, E., Kopecky, J., Mares, M., Schmitt, E., Chmelar, J., Kotsyfakis, M.(2019) Cell Mol Life Sci 76: 2003-2013
- PubMed: 30747251
- DOI: https://doi.org/10.1007/s00018-019-03034-3
- Primary Citation of Related Structures:
5O46 - PubMed Abstract:
To successfully feed, ticks inject pharmacoactive molecules into the vertebrate host including cystatin cysteine protease inhibitors. However, the molecular and cellular events modulated by tick saliva remain largely unknown. Here, we describe and characterize a novel immunomodulatory cystatin, Iristatin, which is upregulated in the salivary glands of feeding Ixodes ricinus ticks. We present the crystal structure of Iristatin at 1.76 Å resolution. Purified recombinant Iristatin inhibited the proteolytic activity of cathepsins L and C and diminished IL-2, IL-4, IL-9, and IFN-γ production by different T-cell populations, IL-6 and IL-9 production by mast cells, and nitric oxide production by macrophages. Furthermore, Iristatin inhibited OVA antigen-induced CD4 + T-cell proliferation and leukocyte recruitment in vivo and in vitro. Our results indicate that Iristatin affects wide range of anti-tick immune responses in the vertebrate host and may be exploitable as an immunotherapeutic.
- Laboratory of Genomics and Proteomics of Disease Vectors, Biology Centre CAS, Institute of Parasitology, Branišovská 1160/31, 37005, České Budějovice, Czech Republic.
Organizational Affiliation: