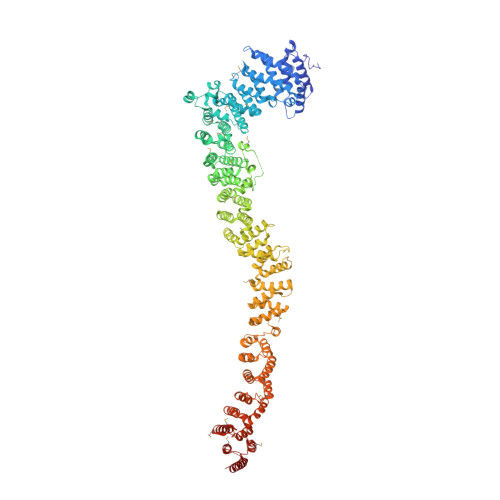
Rif1 maintains telomeres and mediates DNA repair by encasing DNA ends.
Mattarocci, S., Reinert, J.K., Bunker, R.D., Fontana, G.A., Shi, T., Klein, D., Cavadini, S., Faty, M., Shyian, M., Hafner, L., Shore, D., Thoma, N.H., Rass, U.(2017) Nat Struct Mol Biol 24: 588-595
- PubMed: 28604726
- DOI: https://doi.org/10.1038/nsmb.3420
- Primary Citation of Related Structures:
5NVR, 5NW5 - PubMed Abstract:
In yeast, Rif1 is part of the telosome, where it inhibits telomerase and checkpoint signaling at chromosome ends. In mammalian cells, Rif1 is not telomeric, but it suppresses DNA end resection at chromosomal breaks, promoting repair by nonhomologous end joining (NHEJ). Here, we describe crystal structures for the uncharacterized and conserved ∼125-kDa N-terminal domain of Rif1 from Saccharomyces cerevisiae (Rif1-NTD), revealing an α-helical fold shaped like a shepherd's crook. We identify a high-affinity DNA-binding site in the Rif1-NTD that fully encases DNA as a head-to-tail dimer. Engagement of the Rif1-NTD with telomeres proved essential for checkpoint control and telomere length regulation. Unexpectedly, Rif1-NTD also promoted NHEJ at DNA breaks in yeast, revealing a conserved role of Rif1 in DNA repair. We propose that tight associations between the Rif1-NTD and DNA gate access of processing factors to DNA ends, enabling Rif1 to mediate diverse telomere maintenance and DNA repair functions.
- Department of Molecular Biology and Institute of Genetics and Genomics in Geneva (iGE3), University of Geneva, Geneva, Switzerland.
Organizational Affiliation: