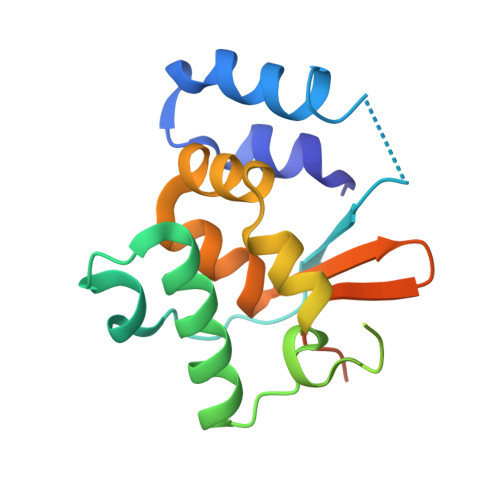
Structure and function of the N-terminal domain of the yeast telomerase reverse transcriptase.
Petrova, O.A., Mantsyzov, A.B., Rodina, E.V., Efimov, S.V., Hackenberg, C., Hakanpaa, J., Klochkov, V.V., Lebedev, A.A., Chugunova, A.A., Malyavko, A.N., Zatsepin, T.S., Mishin, A.V., Zvereva, M.I., Lamzin, V.S., Dontsova, O.A., Polshakov, V.I.(2018) Nucleic Acids Res 46: 1525-1540
- PubMed: 29294091
- DOI: https://doi.org/10.1093/nar/gkx1275
- Primary Citation of Related Structures:
5LGF, 5NPT - PubMed Abstract:
The elongation of single-stranded DNA repeats at the 3'-ends of chromosomes by telomerase is a key process in maintaining genome integrity in eukaryotes. Abnormal activation of telomerase leads to uncontrolled cell division, whereas its down-regulation is attributed to ageing and several pathologies related to early cell death. Telomerase function is based on the dynamic interactions of its catalytic subunit (TERT) with nucleic acids-telomerase RNA, telomeric DNA and the DNA/RNA heteroduplex. Here, we present the crystallographic and NMR structures of the N-terminal (TEN) domain of TERT from the thermotolerant yeast Hansenula polymorpha and demonstrate the structural conservation of the core motif in evolutionarily divergent organisms. We identify the TEN residues that are involved in interactions with the telomerase RNA and in the recognition of the 'fork' at the distal end of the DNA product/RNA template heteroduplex. We propose that the TEN domain assists telomerase biological function and is involved in restricting the size of the heteroduplex during telomere repeat synthesis.
- A.N. Belozersky Institute of Physico-Chemical Biology, M.V. Lomonosov Moscow State University, Moscow 119991, Russia.
Organizational Affiliation: