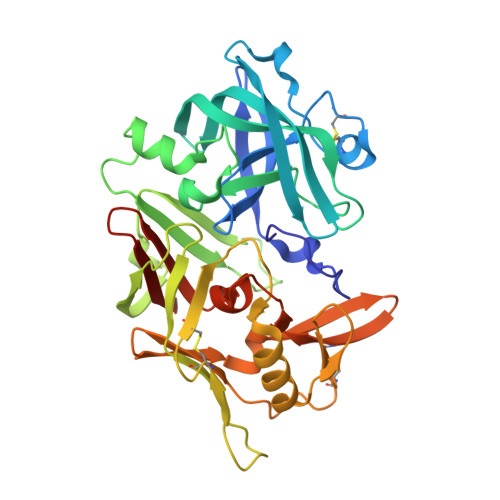
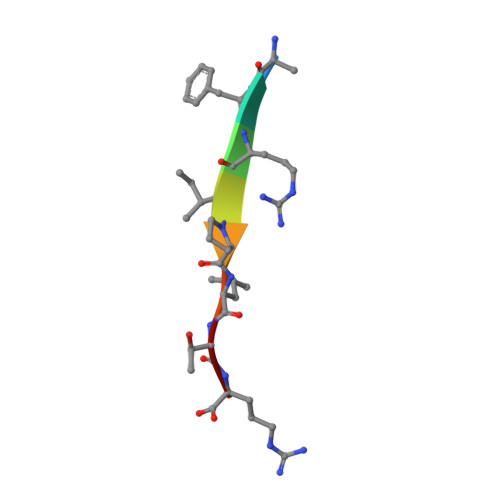
Novel Structural Mechanism of Allosteric Regulation of Aspartic Peptidases via an Evolutionarily Conserved Exosite.
Hanova, I., Brynda, J., Houstecka, R., Alam, N., Sojka, D., Kopacek, P., Maresova, L., Vondrasek, J., Horn, M., Schueler-Furman, O., Mares, M.(2018) Cell Chem Biol 25: 318-329.e4
- PubMed: 29396291
- DOI: https://doi.org/10.1016/j.chembiol.2018.01.001
- Primary Citation of Related Structures:
5N70, 5N71, 5N7N, 5N7Q - PubMed Abstract:
Pepsin-family aspartic peptidases are biosynthesized as inactive zymogens in which the propeptide blocks the active site until its proteolytic removal upon enzyme activation. Here, we describe a novel dual regulatory function for the propeptide using a set of crystal structures of the parasite cathepsin D IrCD1. In the IrCD1 zymogen, intramolecular autoinhibition by the intact propeptide is mediated by an evolutionarily conserved exosite on the enzyme core. After activation, the mature enzyme employs the same exosite to rebind a small fragment derived from the cleaved propeptide. This fragment functions as an effective natural inhibitor of mature IrCD1 that operates in a pH-dependent manner through a unique allosteric inhibition mechanism. The study uncovers the propeptide-binding exosite as a target for the regulation of pepsin-family aspartic peptidases and defines the structural requirements for exosite inhibition.
- Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, 16610 Prague, Czech Republic; Department of Biochemistry, Faculty of Science, Charles University, 12840 Prague, Czech Republic.
Organizational Affiliation: