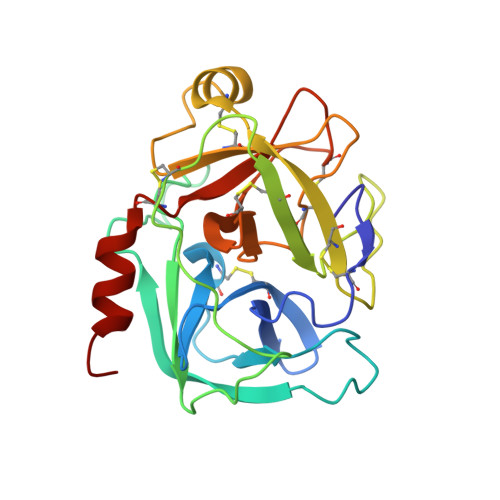
Specificity profiles and antagonistic Ca2+ and Zn2+ regulation of human KLK8/neuropsin activity by modules identified in crystal structures
Debela, M., Magdolen, V., Skala, W., Craik, C.S., Schneider, E.L., Biniossek, M.L., Schilling, O., Elsaesser, B., Bode, W., Brandstetter, H., Goettig, P.To be published.