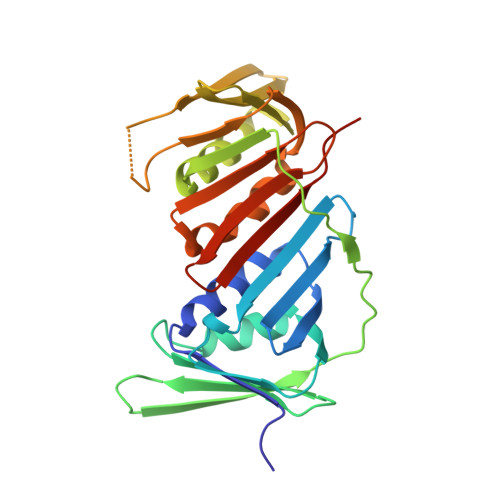
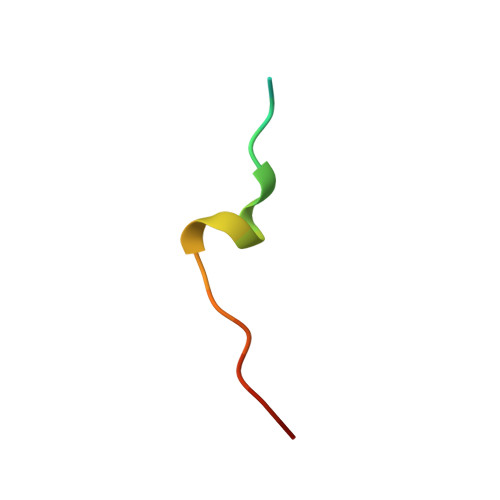
A novel non-canonical PIP-box mediates PARG interaction with PCNA.
Kaufmann, T., Grishkovskaya, I., Polyansky, A.A., Kostrhon, S., Kukolj, E., Olek, K.M., Herbert, S., Beltzung, E., Mechtler, K., Peterbauer, T., Gotzmann, J., Zhang, L., Hartl, M., Zagrovic, B., Elsayad, K., Djinovic-Carugo, K., Slade, D.(2017) Nucleic Acids Res 45: 9741-9759
- PubMed: 28934471
- DOI: https://doi.org/10.1093/nar/gkx604
- Primary Citation of Related Structures:
5MAV - PubMed Abstract:
Poly(ADP-ribose) glycohydrolase (PARG) regulates cellular poly(ADP-ribose) (PAR) levels by rapidly cleaving glycosidic bonds between ADP-ribose units. PARG interacts with proliferating cell nuclear antigen (PCNA) and is strongly recruited to DNA damage sites in a PAR- and PCNA-dependent fashion. Here we identified PARG acetylation site K409 that is essential for its interaction with PCNA, its localization within replication foci and its recruitment to DNA damage sites. We found K409 to be part of a non-canonical PIP-box within the PARG disordered regulatory region. The previously identified putative N-terminal PIP-box does not bind PCNA directly but contributes to PARG localization within replication foci. X-ray structure and MD simulations reveal that the PARG non-canonical PIP-box binds PCNA in a manner similar to other canonical PIP-boxes and may represent a new type of PIP-box. While the binding of previously described PIP-boxes is based on hydrophobic interactions, PARG PIP-box binds PCNA via both stabilizing hydrophobic and fine-tuning electrostatic interactions. Our data explain the mechanism of PARG-PCNA interaction through a new PARG PIP-box that exhibits non-canonical sequence properties but a canonical mode of PCNA binding.
- Department of Biochemistry, Max F. Perutz Laboratories, University of Vienna, Vienna Biocenter (VBC), Dr. Bohr-Gasse 9, 1030 Vienna, Austria.
Organizational Affiliation: