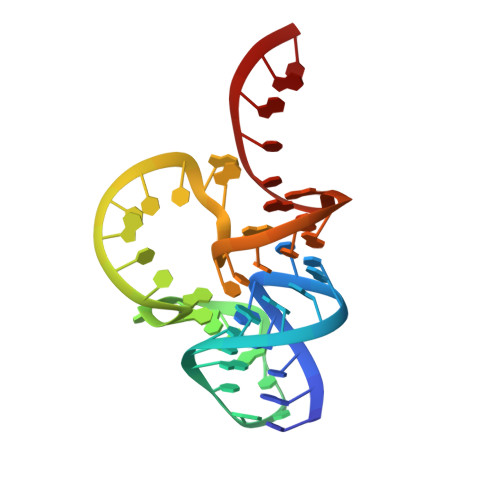
Pistol ribozyme adopts a pseudoknot fold facilitating site-specific in-line cleavage.
Ren, A., Vusurovic, N., Gebetsberger, J., Gao, P., Juen, M., Kreutz, C., Micura, R., Patel, D.J.(2016) Nat Chem Biol 12: 702-708
- PubMed: 27398999
- DOI: https://doi.org/10.1038/nchembio.2125
- Primary Citation of Related Structures:
5K7C, 5K7D, 5K7E - PubMed Abstract:
The field of small self-cleaving nucleolytic ribozymes has been invigorated by the recent discovery of the twister, twister-sister, pistol and hatchet ribozymes. We report the crystal structure of a pistol ribozyme termed env25, which adopts a compact tertiary architecture stabilized by an embedded pseudoknot fold. The G-U cleavage site adopts a splayed-apart conformation with in-line alignment of the modeled 2'-O of G for attack on the adjacent to-be-cleaved P-O5' bond. Highly conserved residues G40 (N1 position) and A32 (N3 and 2'-OH positions) are aligned to act as a general base and a general acid, respectively, to accelerate cleavage chemistry, with their roles confirmed by cleavage assays on variants, and an increased pKa of 4.7 for A32. Our structure of the pistol ribozyme defined how the overall and local topologies dictate the in-line alignment at the G-U cleavage site, with cleavage assays on variants revealing key residues that participate in acid-base-catalyzed cleavage chemistry.
- Life Sciences Institute, Zhejiang University, Hangzhou, China.
Organizational Affiliation: