Structural Basis for the Versatile and Methylation-Dependent Binding of CTCF to DNA.
Hashimoto, H., Wang, D., Horton, J.R., Zhang, X., Corces, V.G., Cheng, X.(2017) Mol Cell 66: 711-720.e3
- PubMed: 28529057
- DOI: https://doi.org/10.1016/j.molcel.2017.05.004
- Primary Citation of Related Structures:
5K5H, 5K5I, 5K5J, 5K5L, 5KKQ, 5T00, 5T0U, 5UND - PubMed Abstract:
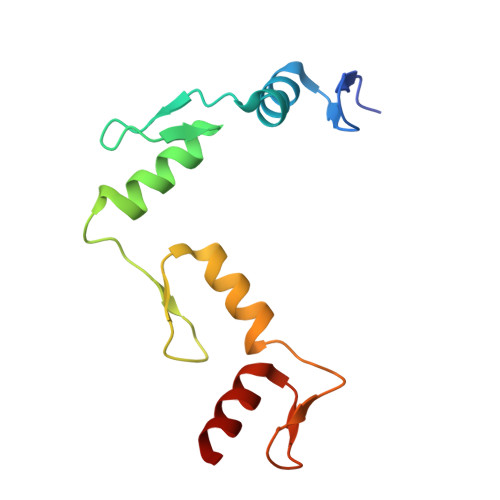
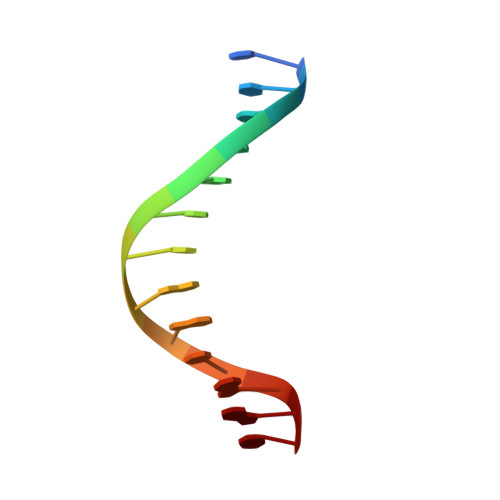
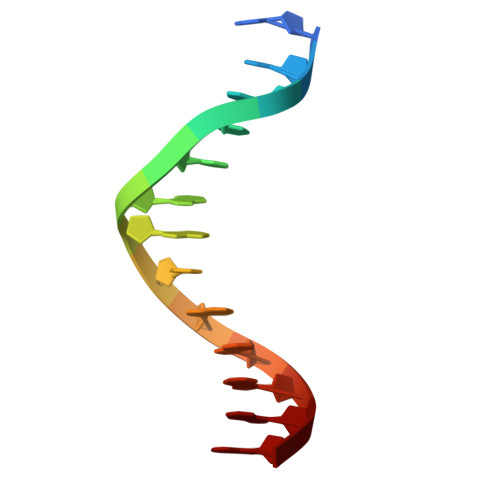
The multidomain CCCTC-binding factor (CTCF), containing a tandem array of 11 zinc fingers (ZFs), modulates the three-dimensional organization of chromatin. We crystallized the human CTCF DNA-binding domain in complex with a known CTCF-binding site. While ZF2 does not make sequence-specific contacts, each finger of ZF3-7 contacts three bases of the 15-bp consensus sequence. Each conserved nucleotide makes base-specific hydrogen bonds with a particular residue. Most of the variable base pairs within the core sequence also engage in interactions with the protein. These interactions compensate for deviations from the consensus sequence, allowing CTCF to adapt to sequence variations. CTCF is sensitive to cytosine methylation at position 2, but insensitive at position 12 of the 15-bp core sequence. These differences can be rationalized structurally. Although included in crystallizations, ZF10 and ZF11 are not visible, while ZF8 and ZF9 span the backbone of the DNA duplex, conferring no sequence specificity but adding to overall binding stability.
- Department of Biochemistry, Emory University School of Medicine, 1510 Clifton Road NE, Atlanta, GA 30322, USA.
Organizational Affiliation: