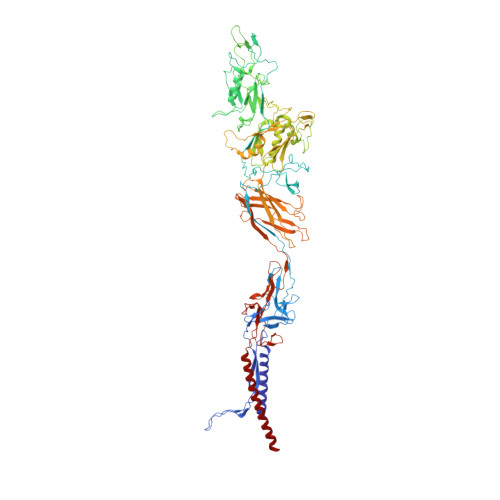
Complete structure of the bacterial flagellar hook reveals extensive set of stabilizing interactions
Matsunami, H., Barker, C.S., Yoon, Y.H., Wolf, M., Samatey, F.A.(2016) Nat Commun 7: 13425-13425
- PubMed: 27811912
- DOI: https://doi.org/10.1038/ncomms13425
- Primary Citation of Related Structures:
5JXL - PubMed Abstract:
The bacterial flagellar hook is a tubular helical structure made by the polymerization of multiple copies of a protein, FlgE. Here we report the structure of the hook from Campylobacter jejuni by cryo-electron microscopy at a resolution of 3.5 Å. On the basis of this structure, we show that the hook is stabilized by intricate inter-molecular interactions between FlgE molecules. Extra domains in FlgE, found only in Campylobacter and in related bacteria, bring more stability and robustness to the hook. Functional experiments suggest that Campylobacter requires an unusually strong hook to swim without its flagella being torn off. This structure reveals details of the quaternary organization of the hook that consists of 11 protofilaments. Previous study of the flagellar filament of Campylobacter by electron microscopy showed its quaternary structure made of seven protofilaments. Therefore, this study puts in evidence the difference between the quaternary structures of a bacterial filament and its hook.
- Trans-Membrane Trafficking Unit, Okinawa Institute of Science and Technology Graduate University, 1919-1 Tancha, Onna, Kunigami 904-0495, Japan.
Organizational Affiliation: