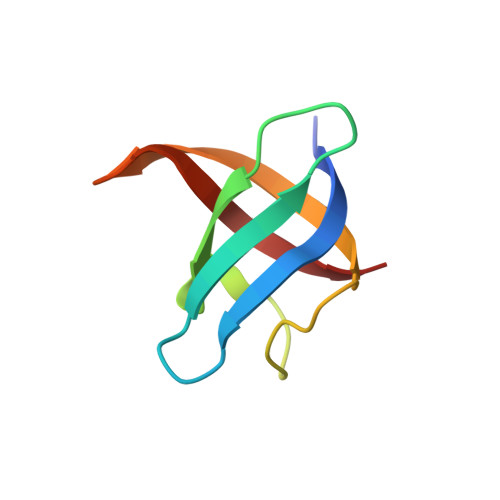
Unusual dimerization of a BcCsp mutant leads to reduced conformational dynamics.
Carvajal, A.I., Vallejos, G., Komives, E.A., Castro-Fernandez, V., Leonardo, D.A., Garratt, R.C., Ramirez-Sarmiento, C.A., Babul, J.(2017) FEBS J 284: 1882-1896
- PubMed: 28457014
- DOI: https://doi.org/10.1111/febs.14093
- Primary Citation of Related Structures:
5JX4 - PubMed Abstract:
Cold shock proteins (Csp) constitute a family of ubiquitous small proteins that act as RNA-chaperones to avoid cold-induced termination of translation. All members contain two subdomains composed of 2 and 3 β-strands, respectively, which are connected by a hinge loop and fold into a β-barrel. Bacillus caldolyticus Csp (BcCsp) is one of the most studied members of the family in terms of its folding, function, and structure. This protein has been described as a monomer in solution, although a recent crystal structure showed dimerization via domain swapping (DS). In contrast, other cold shock proteins of the same fold are known to dimerize in a nonswapped arrangement. Hypothesizing that reducing the size of the hinge loop may promote swapping as in several other DS proteins with different folds we deleted two residues from these region (BcCsp∆36-37), leading to a protein in monomer-dimer equilibrium with similar folding stability to that of the wild-type. Strikingly, the crystal structure of BcCsp∆36-37 revealed a nonswapped dimer with its interface located at the nucleic acid-binding surface, showing that the deletion led to structural consequences far from the perturbation site. Concomitantly, circular dichroism experiments on BcCsp∆36-37 demonstrated that binding of the oligonucleotide hexathymidine disrupts the dimer. Additionally, HDXMS shows a protective effect on the protein structure upon dimerization, where the resulting interactions between ligand-binding surfaces in the dimer reduced the extent of exchange throughout the whole protein. Our work provides evidence of the complex interplay between conformational dynamics, deletions, and oligomerization within the Csp protein family. Structural data are available in the Protein Data Bank under accession number 5JX4.
- Departamento de Biología, Facultad de Ciencias, Universidad de Chile, Santiago, Chile.
Organizational Affiliation: