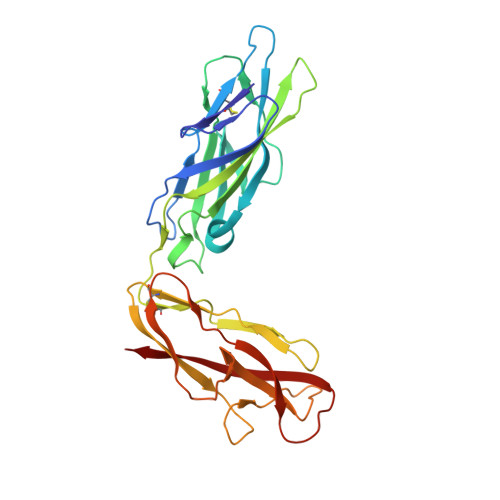
Evolutionary fine-tuning of conformational ensembles in FimH during host-pathogen interactions.
Kalas, V., Pinkner, J.S., Hannan, T.J., Hibbing, M.E., Dodson, K.W., Holehouse, A.S., Zhang, H., Tolia, N.H., Gross, M.L., Pappu, R.V., Janetka, J., Hultgren, S.J.(2017) Sci Adv 3: e1601944-e1601944
- PubMed: 28246638
- DOI: https://doi.org/10.1126/sciadv.1601944
- Primary Citation of Related Structures:
5JQI, 5JR4 - PubMed Abstract:
Positive selection in the two-domain type 1 pilus adhesin FimH enhances Escherichia coli fitness in urinary tract infection (UTI). We report a comprehensive atomic-level view of FimH in two-state conformational ensembles in solution, composed of one low-affinity tense (T) and multiple high-affinity relaxed (R) conformations. Positively selected residues allosterically modulate the equilibrium between these two conformational states, each of which engages mannose through distinct binding orientations. A FimH variant that only adopts the R state is severely attenuated early in a mouse model of uncomplicated UTI but is proficient at colonizing catheterized bladders in vivo or bladder transitional-like epithelial cells in vitro. Thus, the bladder habitat has barrier(s) to R state-mediated colonization possibly conferred by the terminally differentiated bladder epithelium and/or decoy receptors in urine. Together, our studies reveal the conformational landscape in solution, binding mechanisms, and adhesive strength of an allosteric two-domain adhesin that evolved "moderate" affinity to optimize persistence in the bladder during UTI.
- Center for Women's Infectious Disease Research, Department of Molecular Microbiology, Washington University School of Medicine, St. Louis, MO 63110, USA.; Department of Molecular Microbiology, Washington University School of Medicine, St. Louis, MO 63110, USA.; Department of Biochemistry and Molecular Biophysics, Washington University School of Medicine, St. Louis, MO 63110, USA.
Organizational Affiliation: