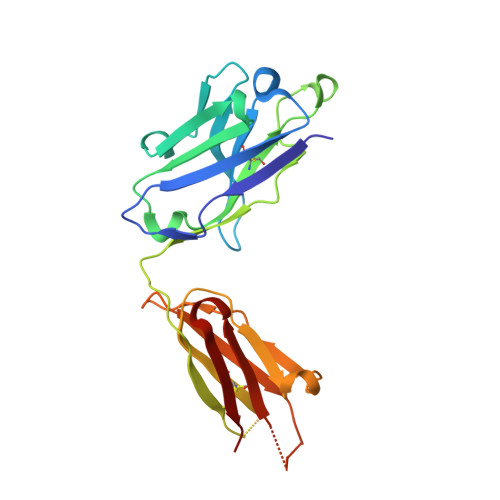
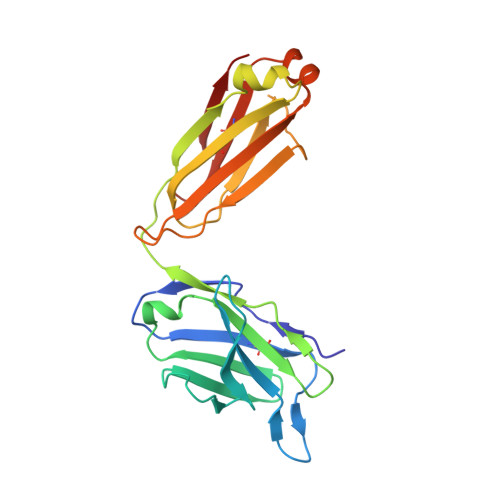
Lessons from the Crystal Structure of the S. aureus Surface Protein Clumping Factor A in Complex With Tefibazumab, an Inhibiting Monoclonal Antibody.
Ganesh, V.K., Liang, X., Geoghegan, J.A., Cohen, A.L., Venugopalan, N., Foster, T.J., Hook, M.(2016) EBioMedicine 13: 328-338
- PubMed: 27789272
- DOI: https://doi.org/10.1016/j.ebiom.2016.09.027
- Primary Citation of Related Structures:
5JQ6 - PubMed Abstract:
The Staphylococcus aureus fibrinogen binding MSCRAMM (Microbial Surface Components Recognizing Adhesive Matrix Molecules), ClfA (clumping factor A) is an important virulence factor in staphylococcal infections and a component of several vaccines currently under clinical evaluation. The mouse monoclonal antibody aurexis (also called 12-9), and the humanized version tefibazumab are therapeutic monoclonal antibodies targeting ClfA that in combination with conventional antibiotics were effective in animal models but showed less impressive efficacy in a limited Phase II clinical trial. We here report the crystal structure and a biochemical characterization of the ClfA/tefibazumab (Fab) complex. The epitope for tefibazumab is located to the "top" of the N3 subdomain of ClfA and partially overlaps with a previously unidentified second binding site for fibrinogen. A high-affinity binding of ClfA to fibrinogen involves both an interaction at the N3 site and the previously identified docking of the C-terminal segment of the fibrinogen γ-chain in the N2N3 trench. Although tefibazumab binds ClfA with high affinity we observe a modest IC 50 value for the inhibition of fibrinogen binding to the MSCRAMM. This observation, paired with a common natural occurring variant of ClfA that is not effectively recognized by the mAb, may partly explain the modest effect tefibazumab showed in the initial clinic trail. This information will provide guidance for the design of the next generation of therapeutic anti-staphylococcal mAbs targeting ClfA.
- Center for Infectious and Inflammatory Diseases, Institute of Biosciences and Technology, Texas A & M University Health Science Center, 2121 W Holcombe Blvd., Houston, TX 77030, USA.
Organizational Affiliation: