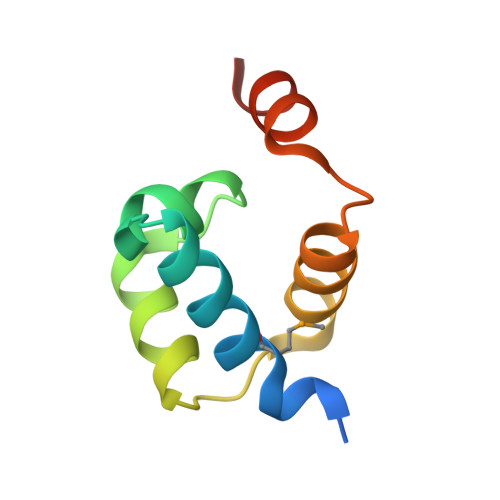
Crystal structure of Pseudomonas aeruginosa RsaL bound to promoter DNA reaffirms its role as a global regulator involved in quorum-sensing.
Kang, H., Gan, J., Zhao, J., Kong, W., Zhang, J., Zhu, M., Li, F., Song, Y., Qin, J., Liang, H.(2017) Nucleic Acids Res 45: 699-710
- PubMed: 27924027
- DOI: https://doi.org/10.1093/nar/gkw954
- Primary Citation of Related Structures:
5J2Y - PubMed Abstract:
Pseudomonas aeruginosa possesses at least three well-defined quorum-sensing (QS) (las, rhl and pqs) systems that control a variety of important functions including virulence. RsaL is a QS repressor that reduces QS signal production and ensures homeostasis by functioning in opposition to LasR. However, its regulatory role in signal homeostasis remains elusive. Here, we conducted a ChIP-seq assay and revealed that RsaL bound to two new targets, the intergenic regions of PA2228/PA2229 and pqsH/cdpR, which are required for PQS synthesis. Deletion of rsaL reduced transcription of pqsH and cdpR, thus decreasing PQS signal production. The ΔrsaL strain exhibited increased pyocyanin production and reduced biofilm formation, which are dependent on CdpR or PqsH activity. In addition, we solved the structure of the RsaL-DNA complex at a 2.4 Å resolution. Although the overall sequence similarity is quite low, RsaL folds into a HTH-like structure, which is conserved among many transcriptional regulators. Complementation results of the rsaL knockout cells with different rsaL mutants further confirmed the critical role of the DNA-binding residues (including Arg20, Gln27, Gln38, Gly35, Ser37 and Ser42) that are essential for DNA binding. Our findings reveal new targets of RsaL and provide insight into the detailed characterization of the RsaL-DNA interaction.
- Key Laboratory of Resources Biology and Biotechnology in Western China, Ministry of Education, College of Life Science, Northwest University, Xi'an, Shaanxi 710069, China.
Organizational Affiliation: