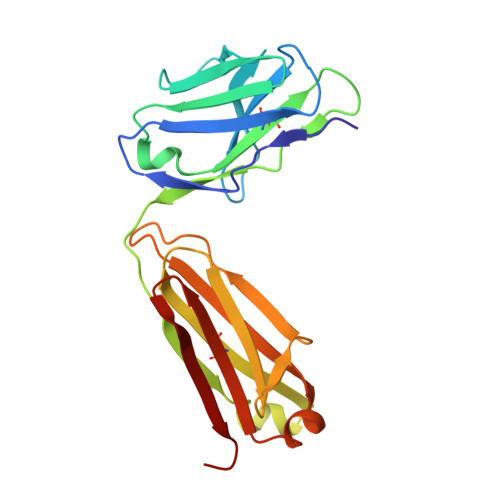
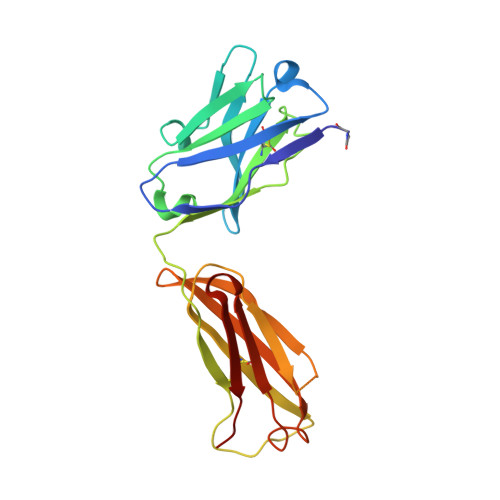
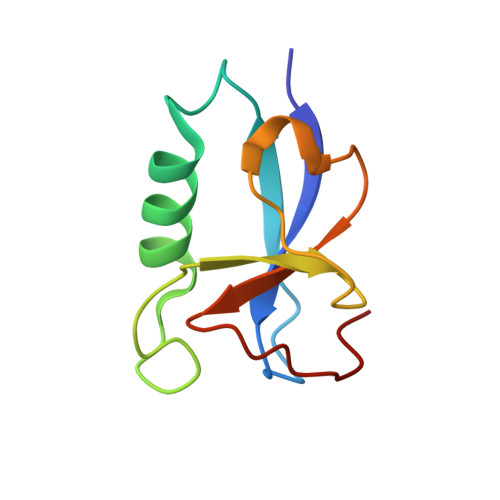
Crystal Structures of the Human Doublecortin C- and N-terminal Domains in Complex with Specific Antibodies.
Burger, D., Stihle, M., Sharma, A., Di Lello, P., Benz, J., D'Arcy, B., Debulpaep, M., Fry, D., Huber, W., Kremer, T., Laeremans, T., Matile, H., Ross, A., Rufer, A.C., Schoch, G., Steinmetz, M.O., Steyaert, J., Rudolph, M.G., Thoma, R., Ruf, A.(2016) J Biological Chem 291: 16292-16306
- PubMed: 27226599
- DOI: https://doi.org/10.1074/jbc.M116.726547
- Primary Citation of Related Structures:
5IKC, 5IN7, 5IO9, 5IOI, 5IP4 - PubMed Abstract:
Doublecortin is a microtubule-associated protein produced during neurogenesis. The protein stabilizes microtubules and stimulates their polymerization, which allows migration of immature neurons to their designated location in the brain. Mutations in the gene that impair doublecortin function and cause severe brain formation disorders are located on a tandem repeat of two doublecortin domains. The molecular mechanism of action of doublecortin is only incompletely understood. Anti-doublecortin antibodies, such as the rabbit polyclonal Abcam 18732, are widely used as neurogenesis markers. Here, we report the generation and characterization of antibodies that bind to single doublecortin domains. The antibodies were used as tools to obtain structures of both domains. Four independent crystal structures of the N-terminal domain reveal several distinct open and closed conformations of the peptide linking N- and C-terminal domains, which can be related to doublecortin function. An NMR assignment and a crystal structure in complex with a camelid antibody fragment show that the doublecortin C-terminal domain adopts the same well defined ubiquitin-like fold as the N-terminal domain, despite its reported aggregation and molten globule-like properties. The antibodies' unique domain specificity also renders them ideal research tools to better understand the role of individual domains in doublecortin function. A single chain camelid antibody fragment specific for the C-terminal doublecortin domain affected microtubule binding, whereas a monoclonal mouse antibody specific for the N-terminal domain did not. Together with steric considerations, this suggests that the microtubule-interacting doublecortin domain observed in cryo-electron micrographs is the C-terminal domain rather than the N-terminal one.
- From the pRED Pharma Research and Early Development, Therapeutic Modalities, and.
Organizational Affiliation: