Inversion of the Side-Chain Stereochemistry of Indvidual Thr or Ile Residues in a Protein Molecule: Impact on the Folding, Stability, and Structure of the ShK Toxin.
Dang, B., Shen, R., Kubota, T., Mandal, K., Bezanilla, F., Roux, B., Kent, S.B.(2017) Angew Chem Int Ed Engl 56: 3324-3328
- PubMed: 28194851
- DOI: https://doi.org/10.1002/anie.201612398
- Primary Citation of Related Structures:
5I5A, 5I5B, 5I5C - PubMed Abstract:
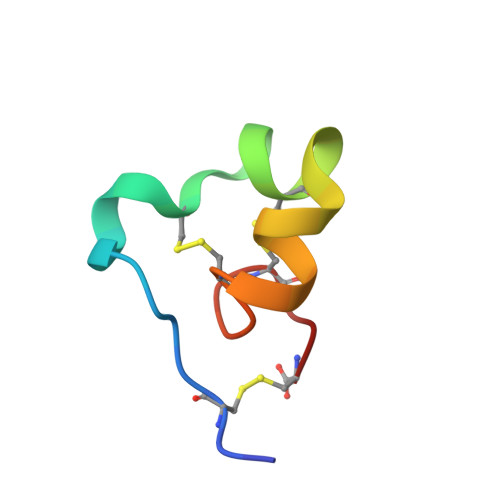
ShK toxin is a cysteine-rich 35-residue protein ion-channel ligand isolated from the sea anemone Stichodactyla helianthus. In this work, we studied the effect of inverting the side chain stereochemistry of individual Thr or Ile residues on the properties of the ShK protein. Molecular dynamics simulations were used to calculate the free energy cost of inverting the side-chain stereochemistry of individual Thr or Ile residues. Guided by the computational results, we used chemical protein synthesis to prepare three ShK polypeptide chain analogues, each containing either an allo-Thr or an allo-Ile residue. The three allo-Thr or allo-Ile-containing ShK polypeptides were able to fold into defined protein products, but with different folding propensities. Their relative thermal stabilities were measured and were consistent with the MD simulation data. Structures of the three ShK analogue proteins were determined by quasi-racemic X-ray crystallography and were similar to wild-type ShK. All three ShK analogues retained ion-channel blocking activity.
- Department of Chemistry, Department of Biochemistry & Molecular Biology, Institute for Biophysical Dynamics, University of Chicago, Chicago, IL, 60637, USA.
Organizational Affiliation: