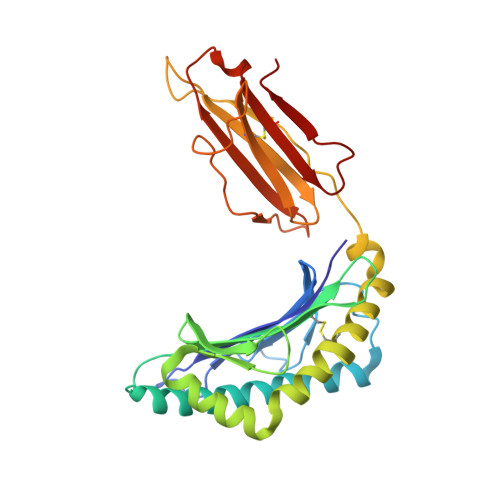
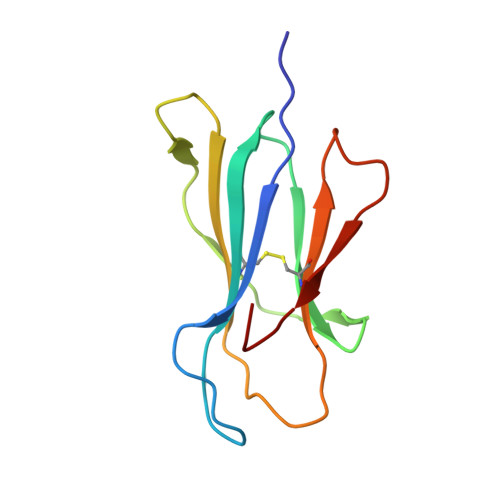
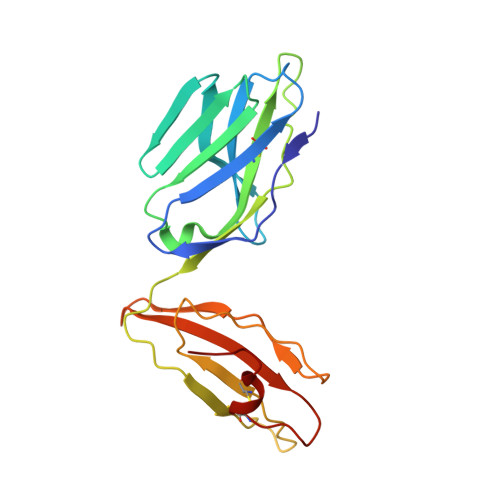
A "hotspot" for autoimmune T cells in type 1 diabetes.
Stadinski, B.D., Obst, R., Huseby, E.S.(2016) J Clin Invest 126: 2040-2042
- PubMed: 27183386
- DOI: https://doi.org/10.1172/JCI88165
- Primary Citation of Related Structures:
5HYJ - PubMed Abstract:
The ability of a single T cell antigen receptor (TCR) to cross-react with multiple antigens allows the finite number of T cells within an organism to respond to the compendium of pathogen challenges faced during a lifetime. Effective immune surveillance, however, comes at a price. TCR cross-reactivity can allow molecular mimics to spuriously activate autoimmune T cells; it also underlies T cell rejection of organ transplants and drives graft-versus-host disease. In this issue of the JCI, Cole and colleagues provide insight into how an insulin-reactive T cell cross-reacts with pathogen-derived antigens by focusing on a limited portion of the peptides to provide a hotspot for binding. These findings dovetail with recent studies of alloreactive and autoimmune TCRs and suggest that the biochemical principles that govern conventional protein-protein interactions may allow the specificity and cross-reactivity profiles of T cells to be predicted.