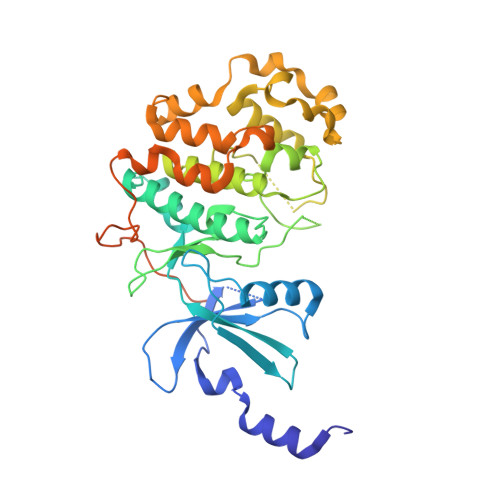
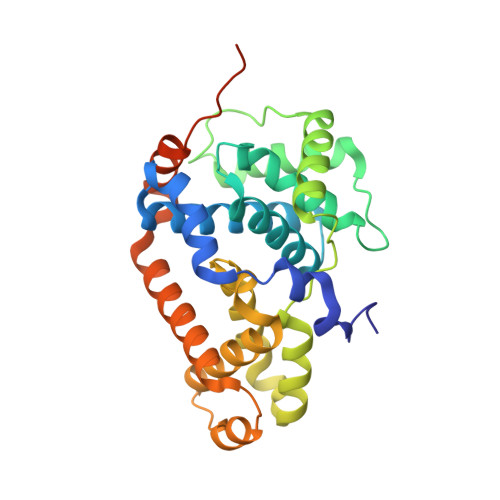
Design and Development of a Series of Potent and Selective Type II Inhibitors of CDK8.
Bergeron, P., Koehler, M.F., Blackwood, E.M., Bowman, K., Clark, K., Firestein, R., Kiefer, J.R., Maskos, K., McCleland, M.L., Orren, L., Ramaswamy, S., Salphati, L., Schmidt, S., Schneider, E.V., Wu, J., Beresini, M.(2016) ACS Med Chem Lett 7: 595-600
- PubMed: 27326333
- DOI: https://doi.org/10.1021/acsmedchemlett.6b00044
- Primary Citation of Related Structures:
5HVY - PubMed Abstract:
Using Sorafenib as a starting point, a series of potent and selective inhibitors of CDK8 was developed. When cocrystallized with CDK8 and cyclin C, these compounds exhibit a Type-II (DMG-out) binding mode.
- Department of Discovery Chemistry, Department of Translational Oncology, Department of Structural Biology, Department of Small Molecule Biochemical Pharmacology, Department of Pathology, Department of Drug Metabolism, and Department of Protein Chemistry, Genentech, Inc. , 1 DNA Way, South San Francisco, California 94080, United States.
Organizational Affiliation: