X-Ray structure of H243I mutant of UDP-Galactose 4-epimerase from E.coli:evidence for existence of open and closed active site during catalysis.
Singh, N., Tiwari, P., Phulera, S., Dixit, A., Choudhury, D.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| UDP-glucose 4-epimerase | 344 | Escherichia coli K-12 | Mutation(s): 1 Gene Names: galE, galD, b0759, JW0742 EC: 5.1.3.2 | 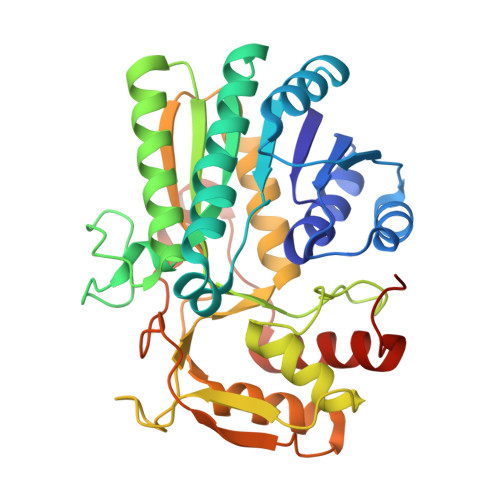 | |
UniProt | |||||
Find proteins for P09147 (Escherichia coli (strain K12)) Explore P09147 Go to UniProtKB: P09147 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P09147 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 4 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| NAD Query on NAD | D [auth A], G [auth B] | NICOTINAMIDE-ADENINE-DINUCLEOTIDE C21 H27 N7 O14 P2 BAWFJGJZGIEFAR-NNYOXOHSSA-N |  | ||
| UDP Query on UDP | C [auth A], F [auth B] | URIDINE-5'-DIPHOSPHATE C9 H14 N2 O12 P2 XCCTYIAWTASOJW-XVFCMESISA-N |  | ||
| GOL Query on GOL | E [auth A] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| NO3 Query on NO3 | H [auth B] | NITRATE ION N O3 NHNBFGGVMKEFGY-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 47.11 | α = 90 |
| b = 91.16 | β = 99.87 |
| c = 80.26 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| MOSFLM | data reduction |
| SCALA | data scaling |
| MOLREP | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Department of Biotechnology | India | DBT- Builder BT/PR5006/INF/22/153/2012 |