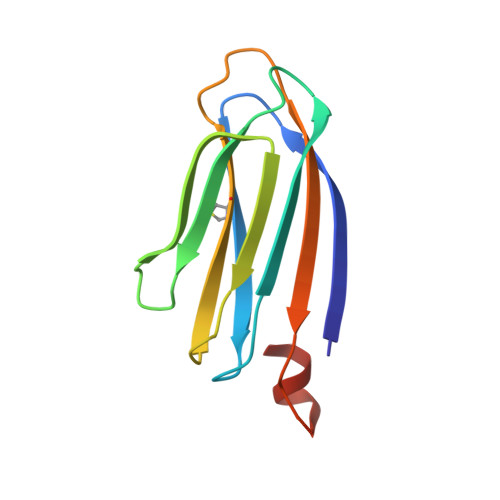
Crystallographic analysis of murine p24 gamma 2 Golgi dynamics domain
Nagae, M., Liebschner, D., Yamada, Y., Morita-Matsumoto, K., Matsugaki, N., Senda, T., Fujita, M., Kinoshita, T., Yamaguchi, Y.(2017) Proteins 85: 764-770
- PubMed: 28066915
- DOI: https://doi.org/10.1002/prot.25242
- Primary Citation of Related Structures:
5GU5 - PubMed Abstract:
The p24 family proteins form homo- and hetero-oligomeric complexes for efficient transport of cargo proteins from the endoplasmic reticulum to the Golgi apparatus. It consists of four subfamilies (p24α, p24β, p24γ, and p24δ). p24γ2 plays crucial roles in the selective transport of glycosylphosphatidylinositol-anchored proteins. Here, we determined the crystal structure of mouse p24γ2 Golgi dynamics (GOLD) domain at 2.8 Å resolution by the single anomalous diffraction method using intrinsic sulfur atoms. In spite of low sequence identity among p24 family proteins, p24γ2 GOLD domain assumes a β-sandwich fold, similar to that of p24β1 or p24δ1. An additional short α-helix is observed at the C-terminus of the p24γ2 GOLD domain. Intriguingly, p24γ2 GOLD domains crystallize as dimers, and dimer formation seems assisted by the short α-helix. Dimerization modes of GOLD domains are compared among p24 family proteins. Proteins 2017; 85:764-770. © 2016 Wiley Periodicals, Inc.
- Structural Glycobiology Team, Systems Glycobiology Research Group, RIKEN-Max Planck Joint Research Center, RIKEN Global Research Cluster, 2-1 Hirosawa, Wako-City, Saitama, 351-0198, Japan.
Organizational Affiliation: