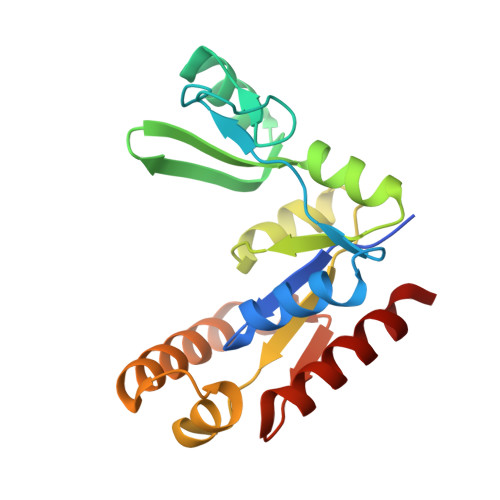
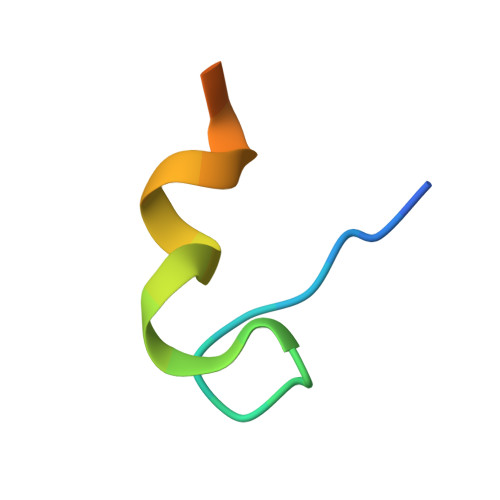
Structure of the PSD-95/MAP1A complex reveals a unique target recognition mode of the MAGUK GK domain
Xia, Y., Shang, Y., Zhang, R., Zhu, J.(2017) Biochem J 474: 2817-2828
- PubMed: 28701415
- DOI: https://doi.org/10.1042/BCJ20170356
- Primary Citation of Related Structures:
5GNV - PubMed Abstract:
The PSD-95 family of membrane-associated guanylate kinases (MAGUKs) are major synaptic scaffold proteins and play crucial roles in the dynamic regulation of dendritic remodelling, which is understood to be the foundation of synaptogenesis and synaptic plasticity. The guanylate kinase (GK) domain of MAGUK family proteins functions as a phosphor-peptide binding module. However, the GK domain of PSD-95 has been found to directly bind to a peptide sequence within the C-terminal region of neuronal-specific microtubule-associated protein 1A (MAP1A), although the detailed molecular mechanism governing this phosphorylation-independent interaction at the atomic level is missing. In the present study, we determine the crystal structure of PSD-95 GK in complex with the MAP1A peptide at 2.6-Å resolution. The complex structure reveals that, unlike a linear and elongated conformation in the phosphor-peptide/GK complexes, the MAP1A peptide adopts a unique conformation with a stretch of hydrophobic residues far from each other in the primary sequence clustering and interacting with the 'hydrophobic site' of PSD-95 GK and a highly conserved aspartic acid of MAP1A (D2117) mimicking the phosphor-serine/threonine in binding to the 'phosphor-site' of PSD-95 GK. We demonstrate that the MAP1A peptide may undergo a conformational transition upon binding to PSD-95 GK. Further structural comparison of known DLG GK-mediated complexes reveals the target recognition specificity and versatility of DLG GKs.
- National Center for Protein Science Shanghai, State Key Laboratory of Molecular Biology, CAS Center for Excellence in Molecular Cell Science, Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, University of Chinese Academy of Sciences, 333 Haike Road, Shanghai 201203, China.
Organizational Affiliation: