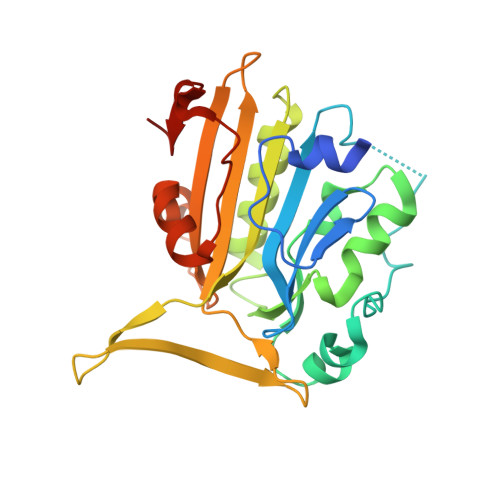
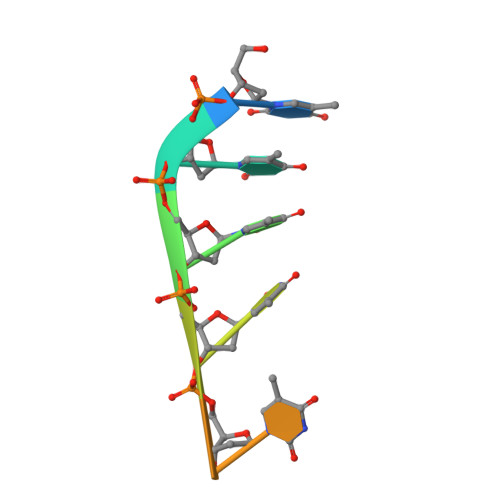
Crystal structure of endonuclease G in complex with DNA reveals how it nonspecifically degrades DNA as a homodimer.
Lin, J.L., Wu, C.C., Yang, W.Z., Yuan, H.S.(2016) Nucleic Acids Res 44: 10480-10490
- PubMed: 27738134
- DOI: https://doi.org/10.1093/nar/gkw931
- Primary Citation of Related Structures:
5GKC, 5GKP - PubMed Abstract:
Endonuclease G (EndoG) is an evolutionarily conserved mitochondrial protein in eukaryotes that digests nucleus chromosomal DNA during apoptosis and paternal mitochondrial DNA during embryogenesis. Under oxidative stress, homodimeric EndoG becomes oxidized and converts to monomers with diminished nuclease activity. However, it remains unclear why EndoG has to function as a homodimer in DNA degradation. Here, we report the crystal structure of the Caenorhabditis elegans EndoG homologue, CPS-6, in complex with single-stranded DNA at a resolution of 2.3 Å. Two separate DNA strands are bound at the ββα-metal motifs in the homodimer with their nucleobases pointing away from the enzyme, explaining why CPS-6 degrades DNA without sequence specificity. Two obligatory monomeric CPS-6 mutants (P207E and K131D/F132N) were constructed, and they degrade DNA with diminished activity due to poorer DNA-binding affinity as compared to wild-type CPS-6. Moreover, the P207E mutant exhibits predominantly 3'-to-5' exonuclease activity, indicating a possible endonuclease to exonuclease activity change. Thus, the dimer conformation of CPS-6 is essential for maintaining its optimal DNA-binding and endonuclease activity. Compared to other non-specific endonucleases, which are usually monomeric enzymes, EndoG is a unique dimeric endonuclease, whose activity hence can be modulated by oxidation to induce conformational changes.
- Institute of Molecular Biology, Academia Sinica, Taipei, Taiwan 11529, ROC.
Organizational Affiliation: