Discovery and Sar of Novel 2,3-Dihydroimidazo(1,2-C)Quinazoline Pi3K Inhibitors: Identification of Copanlisib (Bay 80-6946)
Schaefer, M., Scott, W.J., Hentemann, M.F., Rowley, R.B., Bull, C.O., Jenkins, S., Bullion, A.M., Johnson, J., Redman, A., Robbins, A.H., Esler, W., Fracasso, R.P., Garrison, T., Hamilton, M., Michels, M., Wood, J.E., Wilkie, D.P., Xiao, H., Levy, J., Liu, N., Stasik, E., Brands, M., Lefranc, J.(2016) ChemMedChem 11: 1517
- PubMed: 27310202
- DOI: https://doi.org/10.1002/cmdc.201600148
- Primary Citation of Related Structures:
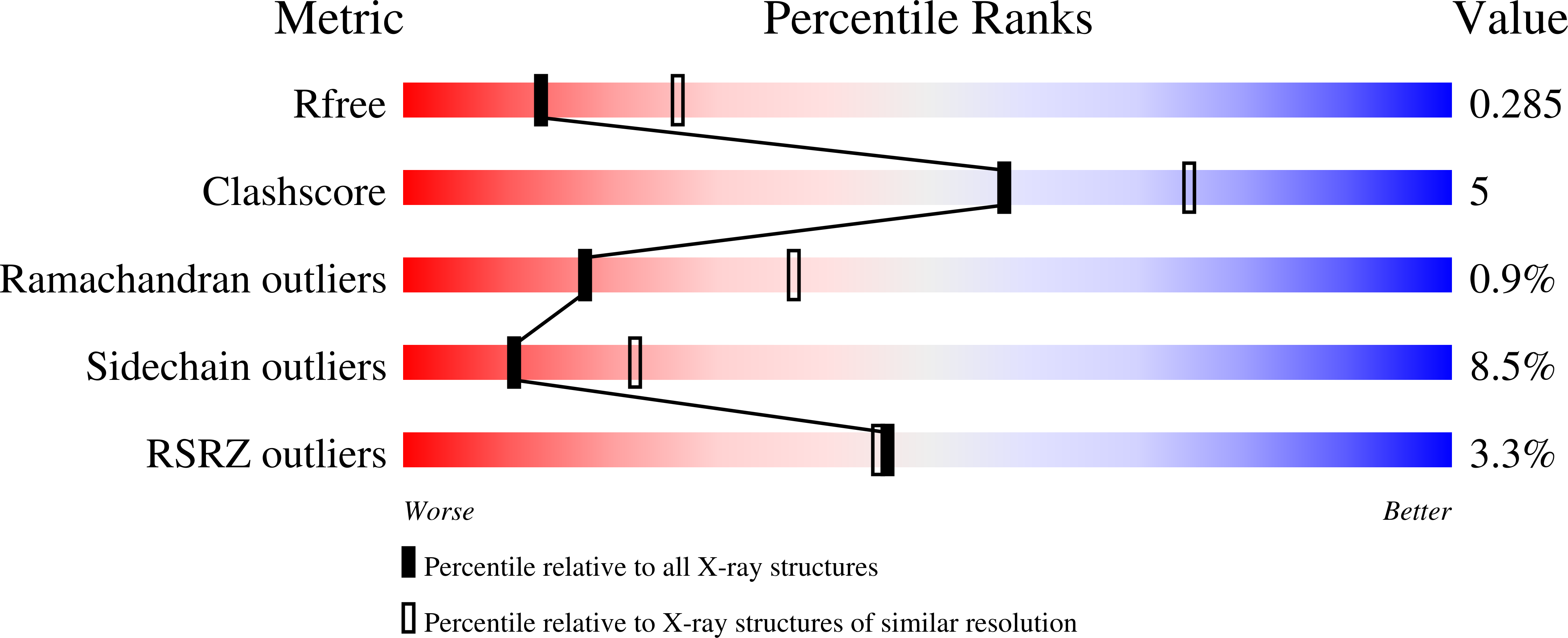
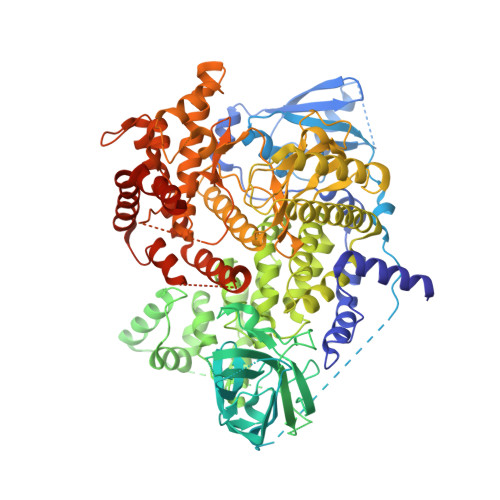
5G2N - PubMed Abstract:
The phosphoinositide 3-kinase (PI3K) pathway is aberrantly activated in many disease states, including tumor cells, either by growth factor receptor tyrosine kinases or by the genetic mutation and amplification of key pathway components. A variety of PI3K isoforms play differential roles in cancers. As such, the development of PI3K inhibitors from novel compound classes should lead to differential pharmacological and pharmacokinetic profiles and allow exploration in various indications, combinations, and dosing regimens. A screening effort aimed at the identification of PI3Kγ inhibitors for the treatment of inflammatory diseases led to the discovery of the novel 2,3-dihydroimidazo[1,2-c]quinazoline class of PI3K inhibitors. A subsequent lead optimization program targeting cancer therapy focused on inhibition of PI3Kα and PI3Kβ. Herein, initial structure-activity relationship findings for this class and the optimization that led to the identification of copanlisib (BAY 80-6946) as a clinical candidate for the treatment of solid and hematological tumors are described.
- Global Development, Global Program Management, Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ, 07981, USA. william.scott@bayer.com.
Organizational Affiliation: