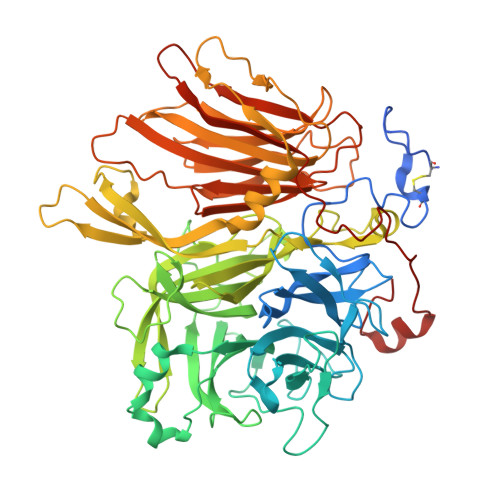
Structural Analysis of Beta-Fructofuranosidase from Xanthophyllomyces Dendrorhous Reveals Unique Features and the Crucial Role of N-Glycosylation in Oligomerization and Activity
Ramirez-Escudero, M., Gimeno-Perez, M., Gonzalez, B., Linde, D., Merdzo, Z., Fernandez-Lobato, M., Sanz-Aparicio, J.(2016) J Biological Chem 291: 6843
- PubMed: 26823463
- DOI: https://doi.org/10.1074/jbc.M115.708495
- Primary Citation of Related Structures:
5ANN, 5FIX, 5FK7, 5FK8, 5FKB, 5FKC, 5FMB, 5FMC, 5FMD - PubMed Abstract:
Xanthophyllomyces dendrorhousβ-fructofuranosidase (XdINV)is a highly glycosylated dimeric enzyme that hydrolyzes sucrose and releases fructose from various fructooligosaccharides (FOS) and fructans. It also catalyzes the synthesis of FOS, prebiotics that stimulate the growth of beneficial bacteria in human gut. In contrast to most fructosylating enzymes, XdINV produces neo-FOS, which makes it an interesting biotechnology target. We present here its three-dimensional structure, which shows the expected bimodular arrangement and also a long extension of its C terminus that together with anN-linked glycan mediate the formation of an unusual dimer. The two active sites of the dimer are connected by a long crevice, which might indicate its potential ability to accommodate branched fructans. This arrangement could be representative of a group of GH32 yeast enzymes having the traits observed in XdINV. The inactive D80A mutant was used to obtain complexes with relevant substrates and products, with their crystals structures showing at least four binding subsites at each active site. Moreover, two different positions are observed from subsite +2 depending on the substrate, and thus, a flexible loop (Glu-334-His-343) is essential in binding sucrose and β(2-1)-linked oligosaccharides. Conversely, β(2-6) and neo-type substrates are accommodated mainly by stacking to Trp-105, explaining the production of neokestose and the efficient fructosylating activity of XdINV on α-glucosides. The role of relevant residues has been investigated by mutagenesis and kinetics measurements, and a model for the transfructosylating reaction has been proposed. The plasticity of its active site makes XdINV a valuable and flexible biocatalyst to produce novel bioconjugates.
- From the Department of Crystallography and Structural Biology, Institute of Physical-Chemistry "Rocasolano," Consejo Superior de Investigaciones Científicas, Serrano 119, 28006 Madrid and.
Organizational Affiliation: