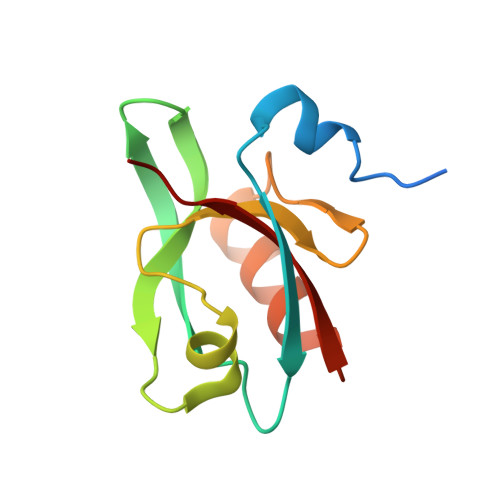
An Exquisitely Specific PDZ/Target Recognition Revealed by the Structure of INAD PDZ3 in Complex with TRP Channel Tail
Ye, F., Liu, W., Shang, Y., Zhang, M.(2016) Structure 24: 383-391
- PubMed: 26853938
- DOI: https://doi.org/10.1016/j.str.2015.12.013
- Primary Citation of Related Structures:
5F67 - PubMed Abstract:
The vast majority of PDZ domains are known to bind to a few C-terminal tail residues of target proteins with modest binding affinities and specificities. Such promiscuous PDZ/target interactions are not compatible with highly specific physiological functions of PDZ domain proteins and their targets. Here, we report an unexpected PDZ/target binding occurring between the scaffold protein inactivation no afterpotential D (INAD) and transient receptor potential (TRP) channel in Drosophila photoreceptors. The C-terminal 15 residues of TRP are required for the specific interaction with INAD PDZ3. The INAD PDZ3/TRP peptide complex structure reveals that only the extreme C-terminal Leu of TRP binds to the canonical αB/βB groove of INAD PDZ3. The rest of the TRP peptide, by forming a β hairpin structure, binds to a surface away from the αB/βB groove of PDZ3 and contributes to the majority of the binding energy. Thus, the INAD PDZ3/TRP channel interaction is exquisitely specific and represents a new mode of PDZ/target recognitions.
- Division of Life Science, State Key Laboratory of Molecular Neuroscience, Hong Kong University of Science and Technology, Clear Water Bay, Hong Kong.
Organizational Affiliation: