Crystal structure of putative aspartate racemase from Salmonella Typhimurium complexed with sulfate and potassium
Maltseva, N., Kim, Y., Stam, J., Anderson, W.F., Joachimiak, A.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Putative aspartate racemase | 268 | Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 | Mutation(s): 0 Gene Names: STM4510 | 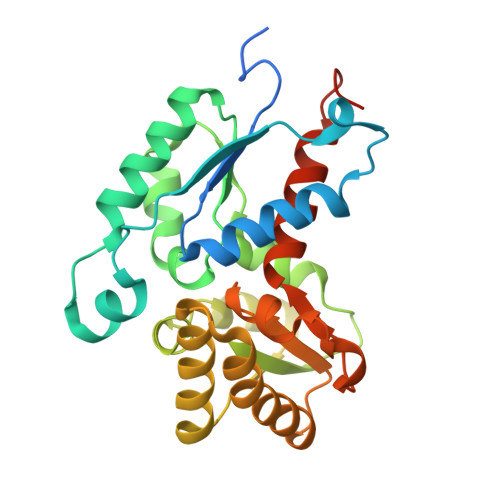 | |
UniProt | |||||
Find proteins for Q8ZJZ9 (Salmonella typhimurium (strain LT2 / SGSC1412 / ATCC 700720)) Explore Q8ZJZ9 Go to UniProtKB: Q8ZJZ9 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q8ZJZ9 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 6 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| SO4 Query on SO4 | D [auth A], J [auth B] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| GOL Query on GOL | F [auth A], L [auth B] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| FMT Query on FMT | G [auth A] | FORMIC ACID C H2 O2 BDAGIHXWWSANSR-UHFFFAOYSA-N |  | ||
| K Query on K | C [auth A], E [auth A], I [auth B], M [auth B] | POTASSIUM ION K NPYPAHLBTDXSSS-UHFFFAOYSA-N |  | ||
| CL Query on CL | H [auth A] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| F Query on F | K [auth B] | FLUORIDE ION F KRHYYFGTRYWZRS-UHFFFAOYSA-M |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 85.328 | α = 90 |
| b = 85.328 | β = 90 |
| c = 113.997 | γ = 120 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-3000 | data reduction |
| HKL-3000 | data scaling |
| HKL-3000 | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute Of Allergy and Infectious Diseases (NIH/NIAID) | United States | -- |