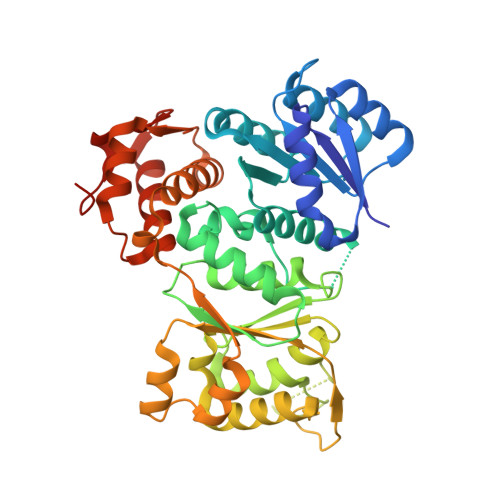
Structure, Regulation, and Inhibition of the Quorum-Sensing Signal Integrator LuxO.
Boyaci, H., Shah, T., Hurley, A., Kokona, B., Li, Z., Ventocilla, C., Jeffrey, P.D., Semmelhack, M.F., Fairman, R., Bassler, B.L., Hughson, F.M.(2016) PLoS Biol 14: e1002464-e1002464
- PubMed: 27219477
- DOI: https://doi.org/10.1371/journal.pbio.1002464
- Primary Citation of Related Structures:
5EP0, 5EP1, 5EP2, 5EP3, 5EP4 - PubMed Abstract:
In a process called quorum sensing, bacteria communicate with chemical signal molecules called autoinducers to control collective behaviors. In pathogenic vibrios, including Vibrio cholerae, the accumulation of autoinducers triggers repression of genes responsible for virulence factor production and biofilm formation. The vibrio autoinducer molecules bind to transmembrane receptors of the two-component histidine sensor kinase family. Autoinducer binding inactivates the receptors' kinase activities, leading to dephosphorylation and inhibition of the downstream response regulator LuxO. Here, we report the X-ray structure of LuxO in its unphosphorylated, autoinhibited state. Our structure reveals that LuxO, a bacterial enhancer-binding protein of the AAA+ ATPase superfamily, is inhibited by an unprecedented mechanism in which a linker that connects the catalytic and regulatory receiver domains occupies the ATPase active site. The conformational change that accompanies receiver domain phosphorylation likely disrupts this interaction, providing a mechanistic rationale for LuxO activation. We also determined the crystal structure of the LuxO catalytic domain bound to a broad-spectrum inhibitor. The inhibitor binds in the ATPase active site and recapitulates elements of the natural regulatory mechanism. Remarkably, a single inhibitor molecule may be capable of inhibiting an entire LuxO oligomer.
- Department of Molecular Biology, Princeton University, Princeton, New Jersey, United States of America.
Organizational Affiliation: