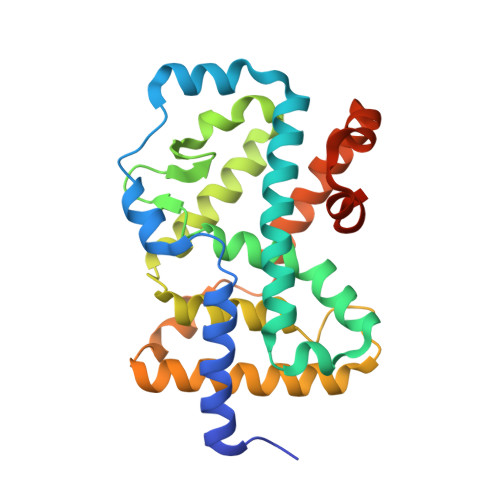
Discovery of biaryls as ROR gamma inverse agonists by using structure-based design.
Enyedy, I.J., Powell, N.A., Caravella, J., van Vloten, K., Chao, J., Banerjee, D., Marcotte, D., Silvian, L., McKenzie, A., Hong, V.S., Fontenot, J.D.(2016) Bioorg Med Chem Lett 26: 2459-2463
- PubMed: 27080181
- DOI: https://doi.org/10.1016/j.bmcl.2016.03.109
- Primary Citation of Related Structures:
5EJV, 5ETH - PubMed Abstract:
RORγ plays a critical role in controlling a pro-inflammatory gene expression program in several lymphocyte lineages including T cells, γδ T cells, and innate lymphoid cells. RORγ-mediated inflammation has been linked to susceptibility to Crohn's disease, arthritis, and psoriasis. Thus inverse agonists of RORγ have the potential of modulating inflammation. Our goal was to optimize two RORγ inverse agonists: T0901317 from literature and 1 that we obtained from internal screening. We used information from internal X-ray structures to design two libraries that led to a new biaryl series.
- Biogen, 250 Binney St., Cambridge, MA 02142, USA. Electronic address: istvan.enyedy@biogen.com.
Organizational Affiliation: