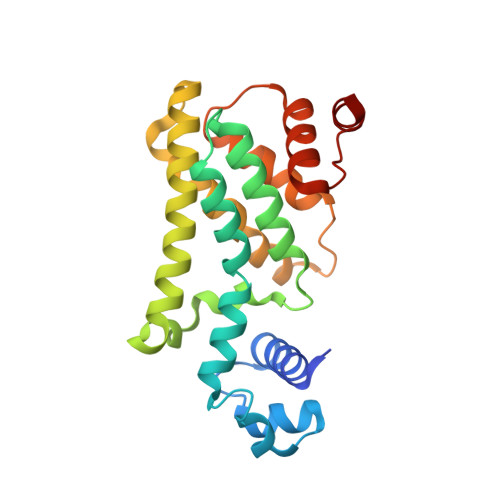
Structure and Function of AmtR in Mycobacterium smegmatis: Implications for Post-Transcriptional Regulation of Urea Metabolism through a Small Antisense RNA.
Petridis, M., Vickers, C., Robson, J., McKenzie, J.L., Bereza, M., Sharrock, A., Aung, H.L., Arcus, V.L., Cook, G.M.(2016) J Mol Biology 428: 4315-4329
- PubMed: 27640309
- DOI: https://doi.org/10.1016/j.jmb.2016.09.009
- Primary Citation of Related Structures:
5E57 - PubMed Abstract:
Soil-dwelling bacteria of the phylum actinomycetes generally harbor either GlnR or AmtR as a global regulator of nitrogen metabolism. Mycobacterium smegmatis harbors both of these canonical regulators; GlnR regulates the expression of key genes involved in nitrogen metabolism, while the function and signal transduction pathway of AmtR in M. smegmatis remains largely unknown. Here, we report the structure and function of the M. smegmatis AmtR and describe the role of AmtR in the regulation of nitrogen metabolism in response to nitrogen availability. To determine the function of AmtR in M. smegmatis, we performed genome-wide expression profiling comparing the wild-type versus an ∆amtR mutant and identified significant changes in the expression of 11 genes, including an operon involved in urea degradation. An AmtR consensus-binding motif (CTGTC-N 4 -GACAG) was identified in the promoter region of this operon, and ligand-independent, high-affinity AmtR binding was validated by both electrophoretic mobility shift assays and surface plasmon resonance measurements. We confirmed the transcription of a cis-encoded small RNA complementary to the gene encoding AmtR under nitrogen excess, and we propose a post-transcriptional regulatory mechanism for AmtR. The three-dimensional X-ray structure of AmtR at 2.0Å revealed an overall TetR-like dimeric structure, and the alignment of the M. smegmatis AmtR and Corynebacterium glutamicum AmtR regulatory domains showed poor structural conservation, providing a potential explanation for the lack of M. smegmatis AmtR interaction with the adenylylated P II protein. Taken together, our data suggest an AmtR (repressor)/GlnR (activator) competitive binding mechanism for transcriptional regulation of urea metabolism that is controlled by a cis-encoded small antisense RNA.
- Department of Microbiology and Immunology, Otago School of Medical Sciences, University of Otago, Dunedin 9054, New Zealand. Electronic address: michael.petridis@otago.ac.nz.
Organizational Affiliation: