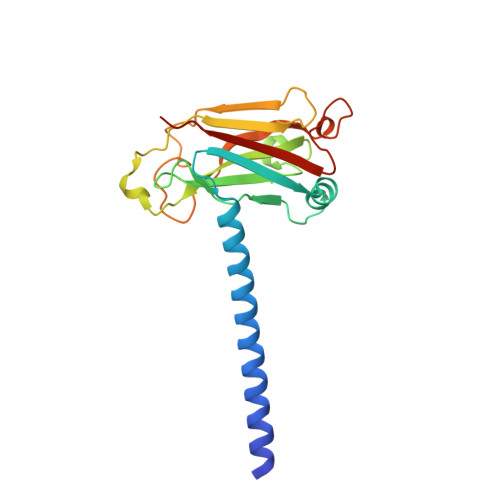
Crystal structure of TRAF1 TRAF domain and its implications in the TRAF1-mediated intracellular signaling pathway
Kim, C.M., Choi, J.Y., Bhat, E.A., Jeong, J.H., Son, Y.J., Kim, S., Park, H.H.(2016) Sci Rep 6: 25526-25526
- PubMed: 27151821
- DOI: https://doi.org/10.1038/srep25526
- Primary Citation of Related Structures:
5E1T - PubMed Abstract:
TNF-receptor associated factor (TRAF) proteins are key adaptor molecules containing E3 ubiquitin ligase activity that play a critical role in immune cell signaling. TRAF1 is a unique family of TRAF lacking the N-terminal RING finger domain. TRAF1 is an important scaffold protein that participates in TNFR2 signaling in T cells as a negative or positive regulator via direct interaction with TRAF2, which has recently been identified as a pro-apoptotic regulator in neuronal cell death. Here, we report the first crystal structure of the TRAF1 TRAF domain containing both the TRAF-N coiled-coil domain and the TRAF-C domain. Our structure reveals both similarities and differences with other TRAF family members, which may be functionally relevant to TRAFs. We also found that the TRAF-N coiled-coil domain of TRAF1 is critical for the trimer formation and stability of the protein. Finally, we found that conserved surface residues on the TRAF1 TRAF domain that might be binding hot spots that are critical for interaction with signaling molecules.
- Department of Biochemistry, Yeungnam University, Gyeongsan, 712-749, South Korea.
Organizational Affiliation: