Structural and Functional Analyses Reveal Insights into the Molecular Properties of the Escherichia coli Z Ring Stabilizing Protein, ZapC.
Schumacher, M.A., Zeng, W., Huang, K.H., Tchorzewski, L., Janakiraman, A.(2016) J Biological Chem 291: 2485-2498
- PubMed: 26655719
- DOI: https://doi.org/10.1074/jbc.M115.697037
- Primary Citation of Related Structures:
5E1L - PubMed Abstract:
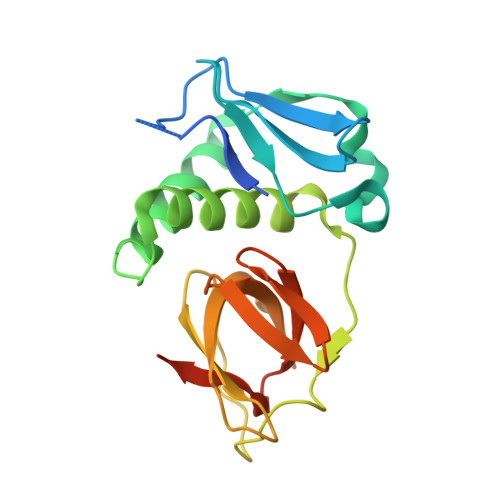
In Escherichia coli cell division is driven by the tubulin-like GTPase, FtsZ, which forms the cytokinetic Z-ring. The Z-ring serves as a dynamic platform for the assembly of the multiprotein divisome, which catalyzes membrane cleavage to create equal daughter cells. Several proteins effect FtsZ assembly, thereby providing spatiotemporal control over cell division. One important class of FtsZ interacting/regulatory proteins is the Z-ring-associated proteins, Zaps, which typically modulate Z-ring formation by increasing lateral interactions between FtsZ protofilaments. Strikingly, these Zap proteins show no discernable sequence similarity, suggesting that they likely harbor distinct structures and mechanisms. The 19.8-kDa ZapC in particular shows no homology to any known protein. To gain insight into ZapC function, we determined its structure to 2.15 Å and performed genetic and biochemical studies. ZapC is a monomer composed of two domains, an N-terminal α/β region and a C-terminal twisted β barrel-like domain. The structure contains two pockets, one on each domain. The N-domain pocket is lined with residues previously implicated to be important for ZapC function as an FtsZ bundler. The adjacent C-domain pocket contains a hydrophobic center surrounded by conserved basic residues. Mutagenesis analyses indicate that this pocket is critical for FtsZ binding. An extensive FtsZ binding surface is consistent with the fact that, unlike many FtsZ regulators, ZapC binds the large FtsZ globular core rather than C-terminal tail, and the presence of two adjacent pockets suggests possible mechanisms for ZapC-mediated FtsZ bundling.
- From the Department of Biochemistry, Duke University School of Medicine, Durham, North Carolina 27710, maria.schumacher@duke.edu.
Organizational Affiliation: