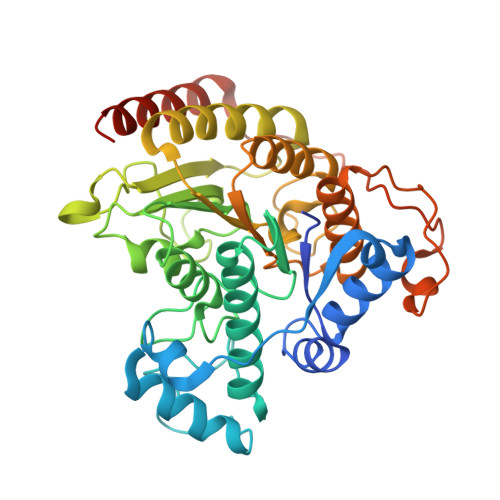
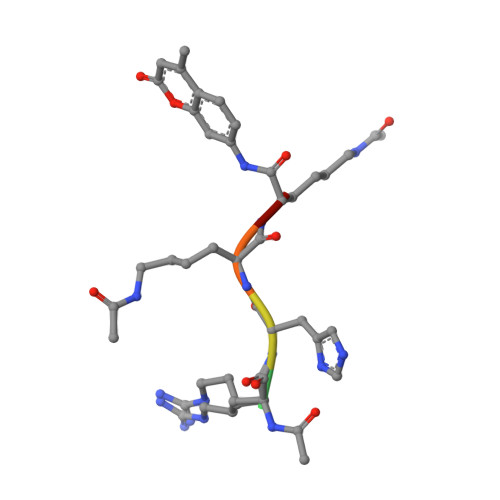
General Base-General Acid Catalysis in Human Histone Deacetylase 8.
Gantt, S.M., Decroos, C., Lee, M.S., Gullett, L.E., Bowman, C.M., Christianson, D.W., Fierke, C.A.(2016) Biochemistry 55: 820-832
- PubMed: 26806311
- DOI: https://doi.org/10.1021/acs.biochem.5b01327
- Primary Citation of Related Structures:
5DC5, 5DC6, 5DC7, 5DC8 - PubMed Abstract:
Histone deacetylases (HDACs) regulate cellular processes such as differentiation and apoptosis and are targeted by anticancer therapeutics in development and in the clinic. HDAC8 is a metal-dependent class I HDAC and is proposed to use a general acid-base catalytic pair in the mechanism of amide bond hydrolysis. Here, we report site-directed mutagenesis and enzymological measurements to elucidate the catalytic mechanism of HDAC8. Specifically, we focus on the catalytic function of Y306 and the histidine-aspartate dyads H142-D176 and H143-D183. Additionally, we report X-ray crystal structures of four representative HDAC8 mutants: D176N, D176N/Y306F, D176A/Y306F, and H142A/Y306F. These structures provide a useful framework for understanding enzymological measurements. The pH dependence of kcat/KM for wild-type Co(II)-HDAC8 is bell-shaped with two pKa values of 7.4 and 10.0. The upper pKa reflects the ionization of the metal-bound water molecule and shifts to 9.1 in Zn(II)-HDAC8. The H142A mutant has activity 230-fold lower than that of wild-type HDAC8, but the pKa1 value is not altered. Y306F HDAC8 is 150-fold less active than the wild-type enzyme; crystal structures show that Y306 hydrogen bonds with the zinc-bound substrate carbonyl, poised for transition state stabilization. The H143A and H142A/H143A mutants exhibit activity that is >80000-fold lower than that of wild-type HDAC8; the buried D176N and D176A mutants have significant catalytic effects, with more subtle effects caused by D183N and D183A. These enzymological and structural studies strongly suggest that H143 functions as a single general base-general acid catalyst, while H142 remains positively charged and serves as an electrostatic catalyst for transition state stabilization.
- Departments of Chemistry and Biological Chemistry, University of Michigan , Ann Arbor, Michigan 48109, United States.
Organizational Affiliation: