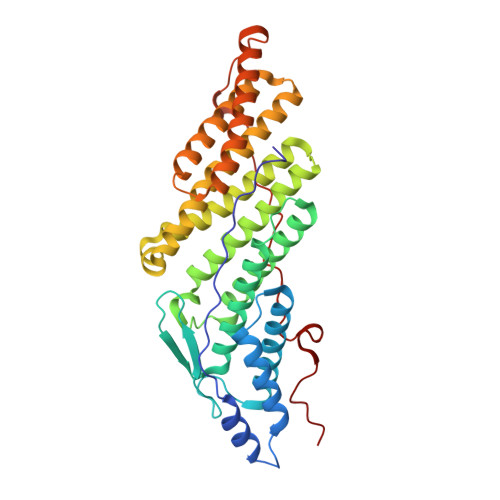
Structural Study of the HD-PTP Bro1 Domain in a Complex with the Core Region of STAM2, a Subunit of ESCRT-0
Lee, J., Oh, K.-J., Lee, D., Kim, B.Y., Choi, J.S., Ku, B., Kim, S.J.(2016) PLoS One 11: e0149113-e0149113
- PubMed: 26866605
- DOI: https://doi.org/10.1371/journal.pone.0149113
- Primary Citation of Related Structures:
5CRU, 5CRV - PubMed Abstract:
EGFR is a key player in cell proliferation and survival signaling, and its sorting into MVBs for eventual lysosomal degradation is controlled by the coordination of multiple ESCRT complexes on the endosomal membrane. HD-PTP is a cytosolic protein tyrosine phosphatase, and is associated with EGFR trafficking by interacting with the ESCRT-0 protein STAM2 and the ESCRT-III protein CHMP4B via its N-terminal Bro1 domain. Intriguingly, the homologous domain of two other human Bro1 domain-containing proteins, Alix and Brox, binds CHMP4B but not STAM2, despite their high structural similarity. To elucidate this binding specificity, we determined the complex structure of the HD-PTP Bro1 domain bound to the STAM2 core region. STAM2 binds to the hydrophobic concave pocket of the HD-PTP Bro1 domain, as CHMP4B does to the pocket of Alix, Brox, or HD-PTP but in the opposite direction. Critically, Thr145 of HD-PTP, corresponding to Lys151 of Alix and Arg145 of Brox, is revealed to be a determinant residue enabling this protein to bind STAM2, as the Alix- or Brox-mimicking mutations of this residue blocks the intermolecular interaction. This work therefore provides the structural basis for how HD-PTP recognizes the ESCRT-0 component to control EGFR sorting.
- Disease Target Structure Research Center, Korea Research Institute of Bioscience and Biotechnology, Daejeon, Korea.
Organizational Affiliation: