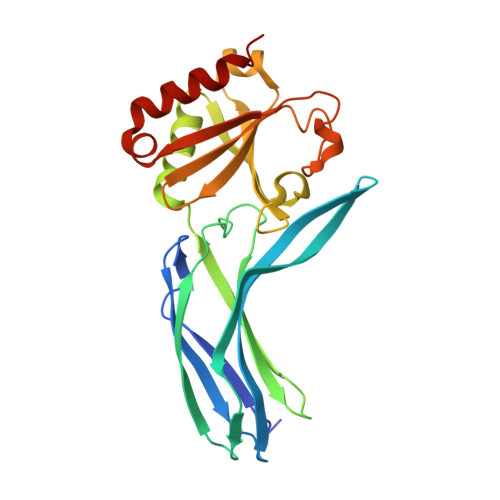
Structure of Spo0M, a sporulation-control protein from Bacillus subtilis.
Sonoda, Y., Mizutani, K., Mikami, B.(2015) Acta Crystallogr F Struct Biol Commun 71: 1488-1497
- PubMed: 26625291
- DOI: https://doi.org/10.1107/S2053230X15020919
- Primary Citation of Related Structures:
5CL2 - PubMed Abstract:
Spo0M is a sporulation-control protein that is thought to play an essential role in the early stage of endospore formation. While little is known about the functions of Spo0M, a recent phylogenetic study suggests that, based on its amino-acid sequence, Spo0M might belong to the arrestin clan. The crystal structure of the Spo0M protein was determined at a resolution of 2.3 Å. Ten amino acids at the end of the N-terminus were removed to improve the thermal stability of the purified Spo0M protein and the crystal structure of Spo0M was determined by SAD. Spo0M has a well conserved N-terminal domain with an arrestin-like fold, which consists of a β-strand sandwich structure. Surprisingly, the C-terminal domain of Spo0M, which has no structural homology to arrestin-clan proteins, bears significant structural similarity to the FP domain of the human PI31 protein. In addition, Spo0M harbours a potential polar-core structure connecting the N- and C-terminal domains with several salt bridges, as seen in the crystal structures of arrestin and VPS26. The structure reported here constitutes the first structural information on a bacterial protein that shares significant structural homology to members of the arrestin clan and the FP domain.
- Laboratory of Applied Structural Biology, Division of Applied Life Sciences, Graduate School of Agriculture, Kyoto University, Uji, Kyoto 611-0011, Japan.
Organizational Affiliation: