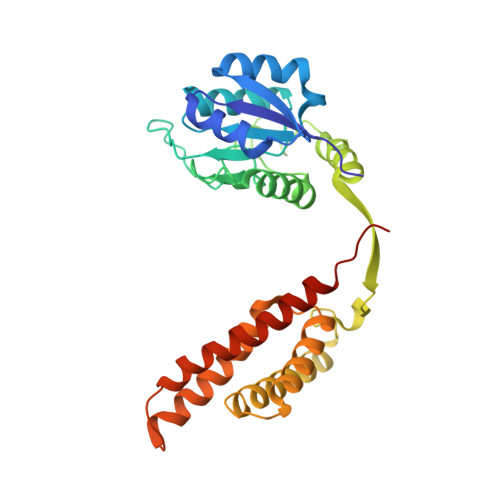
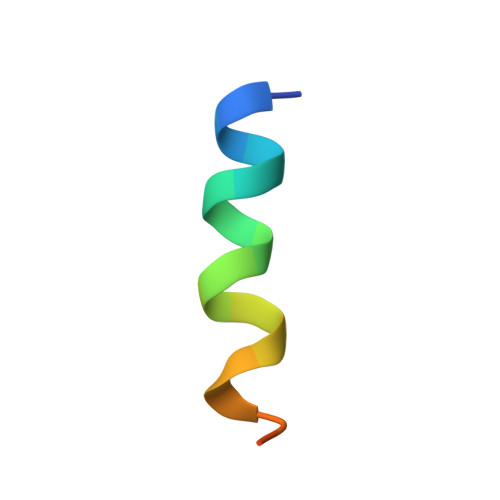
Protein-Protein Interactions in the Cyanobacterial Circadian Clock: Structure of KaiA Dimer in Complex with C-Terminal KaiC Peptides at 2.8 angstrom Resolution.
Pattanayek, R., Egli, M.(2015) Biochemistry 54: 4575-4578
- PubMed: 26200123
- DOI: https://doi.org/10.1021/acs.biochem.5b00694
- Primary Citation of Related Structures:
5C5E - PubMed Abstract:
In the cyanobacterial circadian clock, the KaiA, -B, and -C proteins with ATP constitute a post-translational oscillator. KaiA stimulates the KaiC autokinase, and KaiB antagonizes KaiA action. KaiA contacts the intrinsically disordered C-terminal regions of KaiC hexamer to promote phosphorylation across subunit interfaces. The crystal structure of KaiA dimer from Synechococcus elongatus with two KaiC C-terminal 20mer peptides bound reveals that the latter adopt an α-helical conformation and contact KaiA α-helical bundles via mostly hydrophobic interactions. This complex and the crystal structure of KaiC hexamer with truncated C-terminal tails can be fit into the electron microscopy (EM) density of the KaiA:KaiC complex. The hybrid model helps rationalize clock phenotypes of KaiA and KaiC mutants.
- Department of Biochemistry, Vanderbilt University, School of Medicine, Nashville, Tennessee 37232, United States.
Organizational Affiliation: