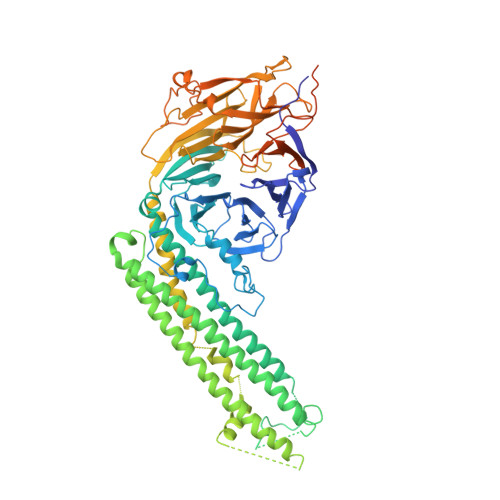
Atomic-Resolution Structures of the APC/C Subunits Apc4 and the Apc5 N-Terminal Domain.
Cronin, N.B., Yang, J., Zhang, Z., Kulkarni, K., Chang, L., Yamano, H., Barford, D.(2015) J Mol Biology 427: 3300-3315
- PubMed: 26343760
- DOI: https://doi.org/10.1016/j.jmb.2015.08.023
- Primary Citation of Related Structures:
5BPT, 5BPW, 5BPZ - PubMed Abstract:
Many essential biological processes are mediated by complex molecular machines comprising multiple subunits. Knowledge on the architecture of individual subunits and their positions within the overall multimeric complex is key to understanding the molecular mechanisms of macromolecular assemblies. The anaphase-promoting complex/cyclosome (APC/C) is a large multisubunit complex that regulates cell cycle progression by ubiquitinating cell cycle proteins for proteolysis by the proteasome. The holo-complex is composed of 15 different proteins that assemble to generate a complex of 20 subunits. Here, we describe the crystal structures of Apc4 and the N-terminal domain of Apc5 (Apc5(N)). Apc4 comprises a WD40 domain split by a long α-helical domain, whereas Apc5(N) has an α-helical fold. In a separate study, we had fitted these atomic models to a 3.6-Å-resolution cryo-electron microscopy map of the APC/C. We describe how, in the context of the APC/C, regions of Apc4 disordered in the crystal assume order through contacts to Apc5, whereas Apc5(N) shows small conformational changes relative to its crystal structure. We discuss the complementary approaches of high-resolution electron microscopy and protein crystallography to the structure determination of subunits of multimeric complexes.
- Division of Structural Biology, Institute of Cancer Research, 237 Fulham Road, London SW3 6JB, United Kingdom.
Organizational Affiliation: