Characterization of the subunit composition and structure of adult human glycine receptors
Yu, H., Bai, X.-C., Wang, W.(2021) Neuron 109: 2707-2716
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
(2021) Neuron 109: 2707-2716
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Glycine receptor subunit alpha-2 | 364 | Homo sapiens | Mutation(s): 0 Gene Names: GLRA2 Membrane Entity: Yes | 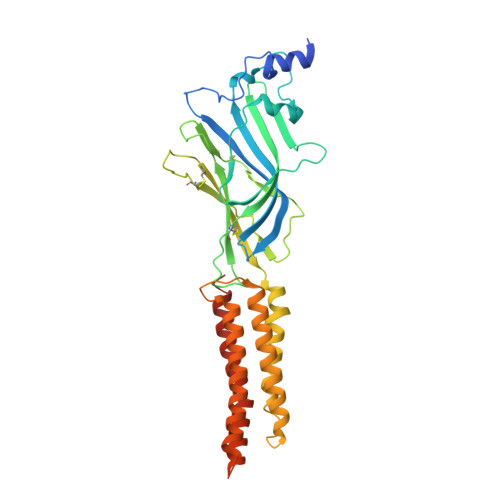 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P23416 (Homo sapiens) Explore P23416 Go to UniProtKB: P23416 | |||||
PHAROS: P23416 GTEx: ENSG00000101958 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P23416 | ||||
Glycosylation | |||||
| Glycosylation Sites: 2 | Go to GlyGen: P23416-1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Glycine receptor subunit beta,Green fluorescent protein | 702 | Homo sapiens, Aequorea victoria | Mutation(s): 0 Gene Names: GLRB, GFP Membrane Entity: Yes | 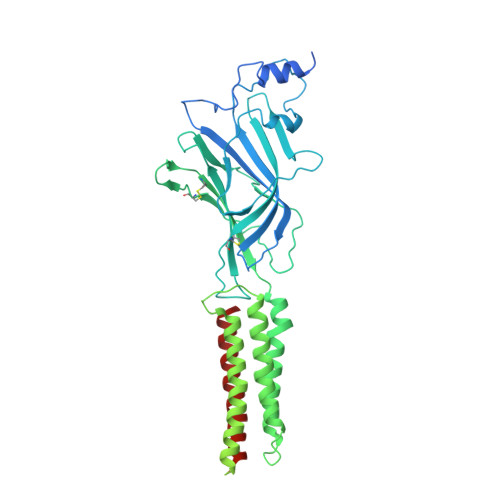 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P42212 (Aequorea victoria) Explore P42212 Go to UniProtKB: P42212 | |||||
Find proteins for P48167 (Homo sapiens) Explore P48167 Go to UniProtKB: P48167 | |||||
PHAROS: P48167 GTEx: ENSG00000109738 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Groups | P42212P48167 | ||||
Glycosylation | |||||
| Glycosylation Sites: 1 | Go to GlyGen: P48167-1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| NAG Query on NAG | F [auth A] G [auth A] I [auth B] J [auth B] M [auth C] | 2-acetamido-2-deoxy-beta-D-glucopyranose C8 H15 N O6 OVRNDRQMDRJTHS-FMDGEEDCSA-N |  | ||
| GLY (Subject of Investigation/LOI) Query on GLY | H [auth A], K [auth B], L [auth B] | GLYCINE C2 H5 N O2 DHMQDGOQFOQNFH-UHFFFAOYSA-N |  | ||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | |
| RECONSTRUCTION | RELION |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Welch Foundation | United States | I-2020-20190330 |