Allosteric Inhibition of a Semaphorin 4D Receptor Plexin B1 by a High-Affinity Macrocyclic Peptide
Matsunaga, Y., Bashiruddin, N.K., Kitago, Y., Takagi, J., Suga, H.(2016) Cell Chem Biol 23: 1341-1350
- PubMed: 27984026
- DOI: https://doi.org/10.1016/j.chembiol.2016.09.015
- Primary Citation of Related Structures:
5B4W - PubMed Abstract:
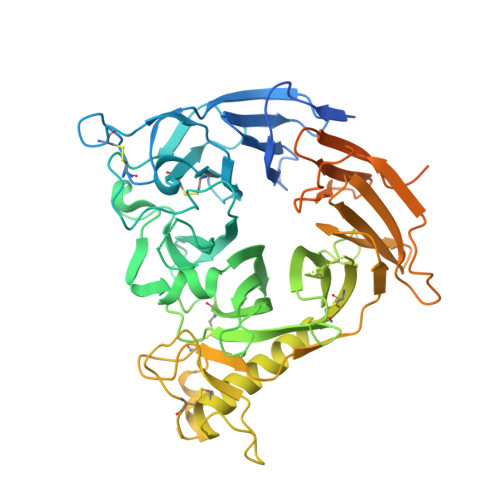
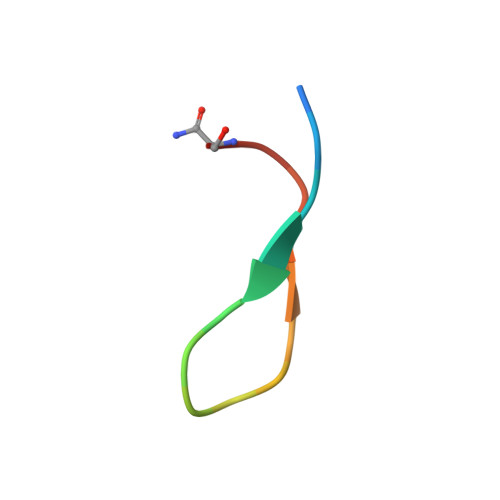
Semaphorin axonal guidance factors are multifunctional proteins that play important roles in immune response, cancer cell proliferation, and organogenesis, making semaphorins and their signaling receptor plexins important drug targets for various diseases. However, the large and flat binding surface of the semaphorin-plexin interaction interface is difficult to target by traditional small-molecule drugs. Here, we report the discovery of a high-affinity plexin B1 (PlxnB1)-binding macrocyclic peptide, PB1m6 (K D = 3.5 nM). PB1m6 specifically inhibited the binding of physiological ligand semaphorin 4D (Sema4D) in vitro and completely suppressed Sema4D-induced cell collapse. Structural studies revealed that PB1m6 binds at a groove between the fifth and sixth blades of the sema domain in PlxnB1 distant from the Sema4D-binding site, indicating the non-competitive and allosteric nature of the inhibitory activity. The discovery of this novel allosteric site can potentially be used to target plexin family proteins for the development of drugs that modulate semaphorin and plexin signaling.
- Laboratory of Protein Synthesis and Expression, Institute for Protein Research, Osaka University, Osaka 565-0871, Japan.
Organizational Affiliation: