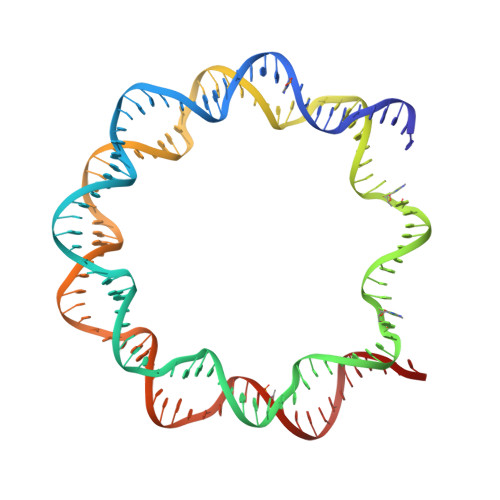
Crystal structure of human nucleosome core particle containing enzymatically introduced CpG methylation.
Fujii, Y., Wakamori, M., Umehara, T., Yokoyama, S.(2016) FEBS Open Bio 6: 498-514
- PubMed: 27419055
- DOI: https://doi.org/10.1002/2211-5463.12064
- Primary Citation of Related Structures:
5B2I, 5B2J - PubMed Abstract:
Cytosine methylation, predominantly of the CpG sequence in vertebrates, is one of the major epigenetic modifications crucially involved in the control of gene expression. Due to the difficulty of reconstituting site-specifically methylated nucleosomal DNA at crystallization quality, most structural analyses of CpG methylation have been performed using chemically synthesized oligonucleotides, There has been just one recent study of nucleosome core particles (NCPs) reconstituted with nonpalindromic human satellite 2-derived DNAs. Through the preparation of a 146-bp palindromic α-satellite-based nucleosomal DNA containing four CpG dinucleotide sequences and its enzymatic methylation and restriction, we reconstituted a 'symmetric' human CpG-methylated nucleosome core particle (NCP). We solved the crystal structures of the CpG-methylated and unmodified NCPs at 2.6 and 3.0 Å resolution, respectively. We observed the electron densities of two methyl groups, among the eight 5-methylcytosines introduced in the CpG-fully methylated NCP. There were no obvious structural differences between the CpG-methylated 'symmetric NCP' and the unmodified NCP. The preparation of a crystallization-grade CpG-methylated NCP provides a platform for the analysis of CpG-methyl reader and eraser proteins.
- RIKEN Systems and Structural Biology Center Tsurumi Yokohama Japan; RIKEN Structural Biology Laboratory Tsurumi Yokohama Japan.
Organizational Affiliation: