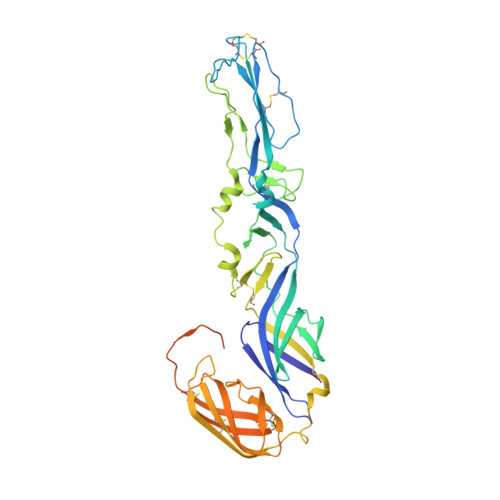
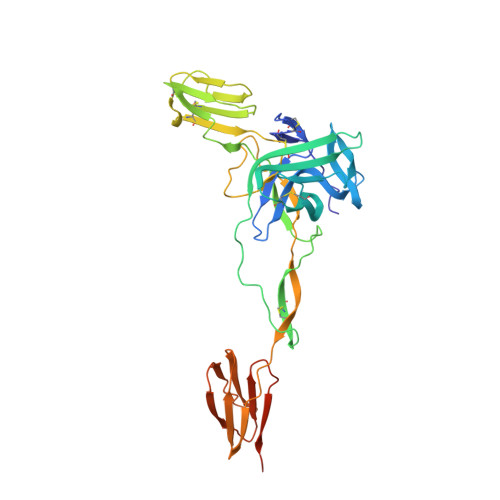
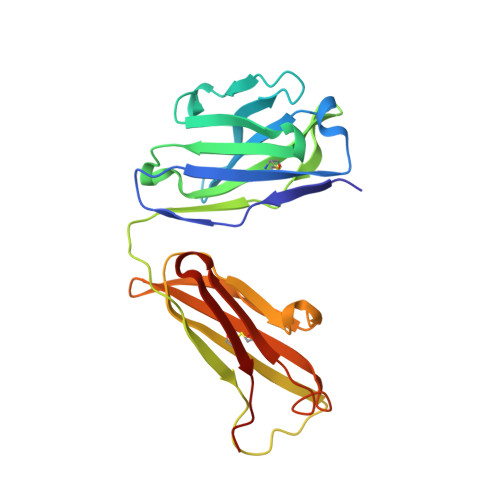
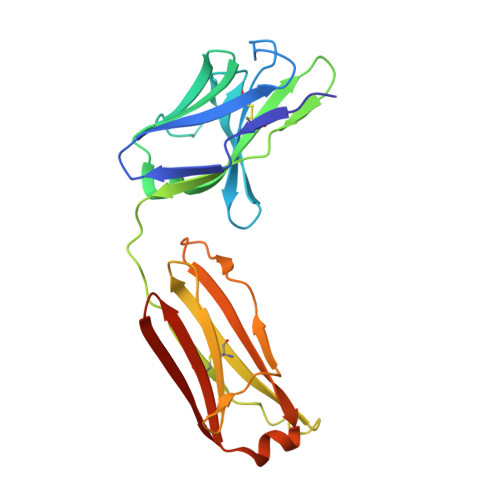
Broadly Neutralizing Alphavirus Antibodies Bind an Epitope on E2 and Inhibit Entry and Egress.
Fox, J.M., Long, F., Edeling, M.A., Lin, H., Van Duijl-Richter, M.K.S., Fong, R.H., Kahle, K.M., Smit, J.M., Jin, J., Simmons, G., Doranz, B.J., Crowe, J.E.J., Fremont, D.H., Rossmann, M.G., Diamond, M.S.(2015) Cell 163: 1095
- PubMed: 26553503
- DOI: https://doi.org/10.1016/j.cell.2015.10.050
- Primary Citation of Related Structures:
5ANY - PubMed Abstract:
We screened a panel of mouse and human monoclonal antibodies (MAbs) against chikungunya virus and identified several with inhibitory activity against multiple alphaviruses. Passive transfer of broadly neutralizing MAbs protected mice against infection by chikungunya, Mayaro, and O'nyong'nyong alphaviruses. Using alanine-scanning mutagenesis, loss-of-function recombinant proteins and viruses, and multiple functional assays, we determined that broadly neutralizing MAbs block multiple steps in the viral lifecycle, including entry and egress, and bind to a conserved epitope on the B domain of the E2 glycoprotein. A 16 Å resolution cryo-electron microscopy structure of a Fab fragment bound to CHIKV E2 B domain provided an explanation for its neutralizing activity. Binding to the B domain was associated with repositioning of the A domain of E2 that enabled cross-linking of neighboring spikes. Our results suggest that B domain antigenic determinants could be targeted for vaccine or antibody therapeutic development against multiple alphaviruses of global concern.
- Department of Medicine, Washington University School of Medicine, St. Louis, MO 63110, USA.
Organizational Affiliation: