Structural and Adhesive Properties of the Long Polar Fimbriae Protein Lpfd from Adherent-Invasive Escherichia Coli.
Coppens, F., Iyyathurai, J., Ruer, S., Fioravanti, A., Taganna, J., Vereecke, L., De Greve, H., Remaut, H.(2015) Acta Crystallogr D Biol Crystallogr 71: 1615
- PubMed: 26249343
- DOI: https://doi.org/10.1107/S1399004715009803
- Primary Citation of Related Structures:
5AFO - PubMed Abstract:
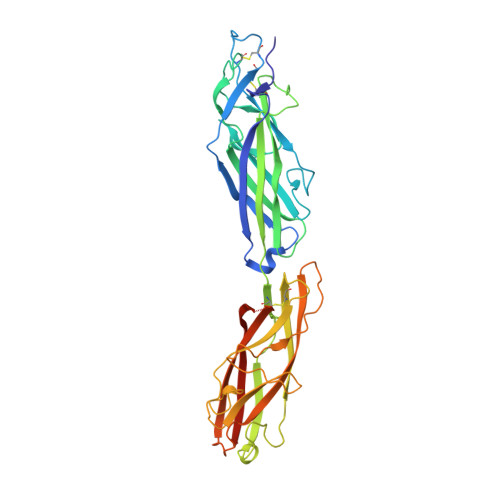
Crohn's disease (CD) is an inflammatory bowel disease characterized by an exaggerated immune response to commensal microbiota in the intestines of patients. Metagenomic studies have identified specific bacterial species and strains with increased prevalence in CD patients, amongst which is the adherent-invasive Escherichia coli (AIEC) strain LF82. AIEC strains express long polar fimbriae (LPF), which are known to target Peyer's patches in a mouse CD model. Here, the recombinant production of a soluble, self-complemented construct of the LpfD protein of E. coli LF82 is reported and it is demonstrated that it forms the adhesive tip subunit of LPF. The LpfD crystal reveals an N-terminal adhesin domain and a C-terminal pilin domain that connects the adhesin to the minor pilus subunit LpfE. Surface topology and sequence conservation in the adhesin domain hint at a putative receptor-binding pocket as found in the Klebsiella pneumoniae MrkD and E. coli F17-G (GafD) adhesins. Immunohistostaining of murine intestinal tissue sections revealed that LpfD specifically binds to the intestinal mucosa and submucosa. LpfD binding was found to be resistant to treatment with O- or N-glycosidases, but was lost in collagenase-treated tissue sections, indicating the possible involvement of an intestinal matrix-associated protein as the LpfD receptor. LpfD strongly adhered to isolated fibronectin in an in vitro assay, and showed lower levels of binding to collagen V and laminin and no binding to collagens I, III and IV.
- Structural and Molecular Microbiology, Structural Biology Research Center, VIB, Pleinlaan 2, 1050 Brussels, Belgium.
Organizational Affiliation: