Metazoan Maelstrom is an RNA-Binding Protein that Has Evolved from an Ancient Nuclease Active in Protists.
Chen, K., Campbell, E., Pandey, R.R., Yang, Z., Mccarthy, A.A., Pillai, R.S.(2015) RNA 21: 833
- PubMed: 25778731
- DOI: https://doi.org/10.1261/rna.049437.114
- Primary Citation of Related Structures:
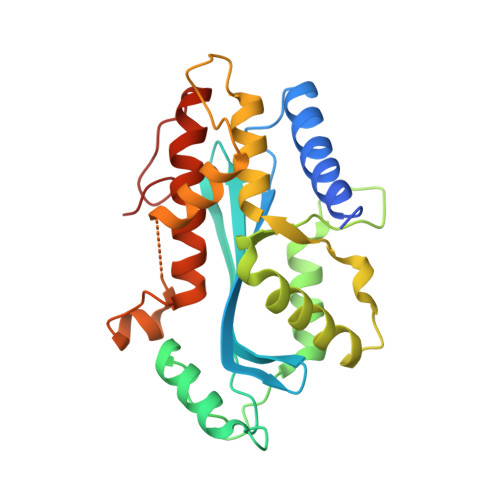
5AF0 - PubMed Abstract:
Piwi-interacting RNAs (piRNAs) guide Piwi argonautes to their transposon targets for silencing. The highly conserved protein Maelstrom is linked to both piRNA biogenesis and effector roles in this pathway. One defining feature of Maelstrom is the predicted MAEL domain of unknown molecular function. Here, we present the first crystal structure of the MAEL domain from Bombyx Maelstrom, which reveals a nuclease fold. The overall architecture resembles that found in Mg(2+)- or Mn(2+)-dependent DEDD nucleases, but a clear distinguishing feature is the presence of a structural Zn(2+) ion coordinated by the conserved ECHC residues. Strikingly, metazoan Maelstrom orthologs across the animal kingdom lack the catalytic DEDD residues, and as we show for Bombyx Maelstrom are inactive as nucleases. However, a MAEL domain-containing protein from amoeba having both sequence motifs (DEDD and ECHC) is robustly active as an exoribonuclease. Finally, we show that the MAEL domain of Bombyx Maelstrom displays a strong affinity for single-stranded RNAs. Our studies suggest that the ancient MAEL nuclease domain evolved to function as an RNA-binding module in metazoan Maelstrom.
- European Molecular Biology Laboratory, Grenoble Outstation, 38042 Grenoble Cedex 9, France Unit for Virus Host-Cell Interactions, University Grenoble Alpes-EMBL-CNRS, 38042 Grenoble Cedex 9, France.
Organizational Affiliation: