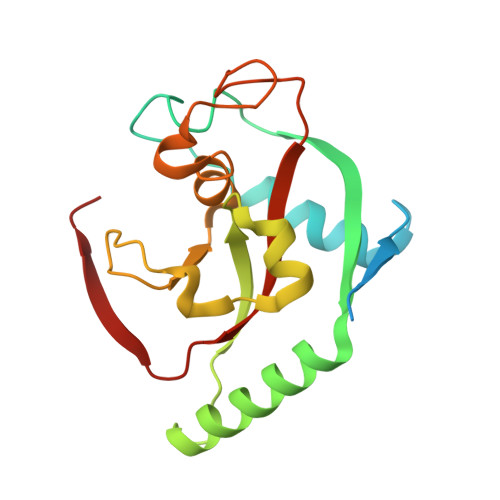
Development and Structural Analysis of Adenosine Site Binding Tankyrase Inhibitors.
Haikarainen, T., Waaler, J., Ignatev, A., Nkizinkiko, Y., Venkannagari, H., Obaji, E., Krauss, S., Lehtio, L.(2016) Bioorg Med Chem Lett 26: 328
- PubMed: 26706174
- DOI: https://doi.org/10.1016/j.bmcl.2015.12.018
- Primary Citation of Related Structures:
5ADQ, 5ADR, 5ADS, 5ADT, 5AEH - PubMed Abstract:
Tankyrases 1 and 2, the specialized members of the ARTD protein family, are druggable biotargets whose inhibition may have therapeutic potential against cancer, metabolic disease, fibrotic disease, fibrotic wound healing and HSV viral infections. We have previously identified a novel tankyrase inhibitor scaffold, JW55, and showed that it reduces mouse colon adenoma formation in vivo. Here we expanded the scaffold and profiled the selectivity of the compounds against a panel of human ARTDs. The scaffold also enables a fine modulation of selectivity towards either tankyrase 1 or tankyrase 2. In order to get insight about the binding mode of the inhibitors, we solved crystal structures of the compounds in complex with tankyrase 2. The compounds bind to the adenosine pocket of the catalytic domain and cause changes in the protein structure that are modulated by the chemical modifications of the compounds. The structural analysis allows further rational development of this compound class as a potent and selective tankyrase inhibitor.
- Faculty of Biochemistry and Molecular Medicine and Biocenter Oulu, University of Oulu, Oulu, Finland.
Organizational Affiliation: