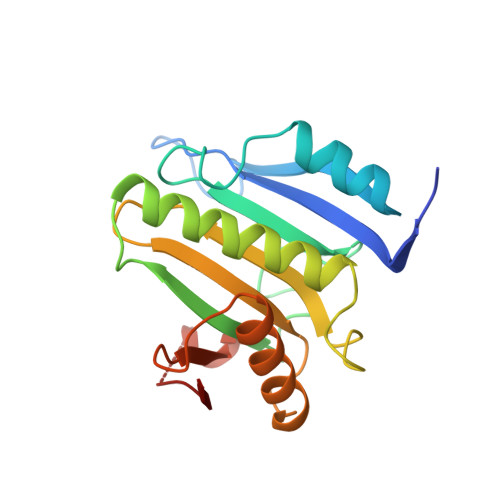
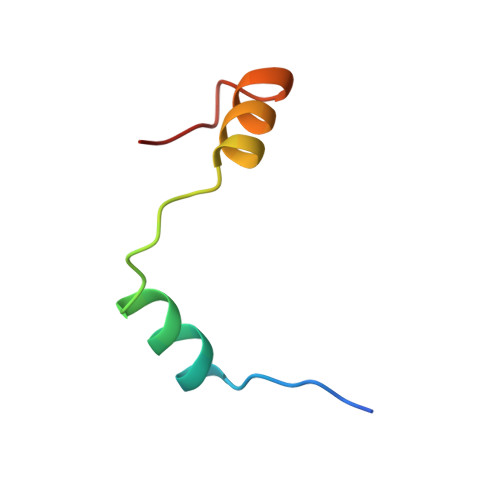
Mextli Proteins Use Both Canonical Bipartite and Novel Tripartite Binding Modes to Form Eif4E Complexes that Display Differential Sensitivity to 4E-BP Regulation
Peter, D., Weber, R., Koene, C., Chung, M.-Y., Ebertsch, L., Truffault, V., Weichenrieder, O., Igreja, C., Izaurralde, E.(2015) Genes Dev 29: 1835
- PubMed: 26294658
- DOI: https://doi.org/10.1101/gad.269068.115
- Primary Citation of Related Structures:
5ABU, 5ABV, 5ABX, 5ABY - PubMed Abstract:
The eIF4E-binding proteins (4E-BPs) are a diverse class of translation regulators that share a canonical eIF4E-binding motif (4E-BM) with eIF4G. Consequently, they compete with eIF4G for binding to eIF4E, thereby inhibiting translation initiation. Mextli (Mxt) is an unusual 4E-BP that promotes translation by also interacting with eIF3. Here we present the crystal structures of the eIF4E-binding regions of the Drosophila melanogaster (Dm) and Caenorhabditis elegans (Ce) Mxt proteins in complex with eIF4E in the cap-bound and cap-free states. The structures reveal unexpected evolutionary plasticity in the eIF4E-binding mode, with a classical bipartite interface for Ce Mxt and a novel tripartite interface for Dm Mxt. Both interfaces comprise a canonical helix and a noncanonical helix that engage the dorsal and lateral surfaces of eIF4E, respectively. Remarkably, Dm Mxt contains a C-terminal auxiliary helix that lies anti-parallel to the canonical helix on the eIF4E dorsal surface. In contrast to the eIF4G and Ce Mxt complexes, the Dm eIF4E-Mxt complexes are resistant to competition by bipartite 4E-BPs, suggesting that Dm Mxt can bind eIF4E when eIF4G binding is inhibited. Our results uncovered unexpected diversity in the binding modes of 4E-BPs, resulting in eIF4E complexes that display differential sensitivity to 4E-BP regulation.
- Department of Biochemistry, Max Planck Institute for Developmental Biology, 72076 Tübingen, Germany.
Organizational Affiliation: