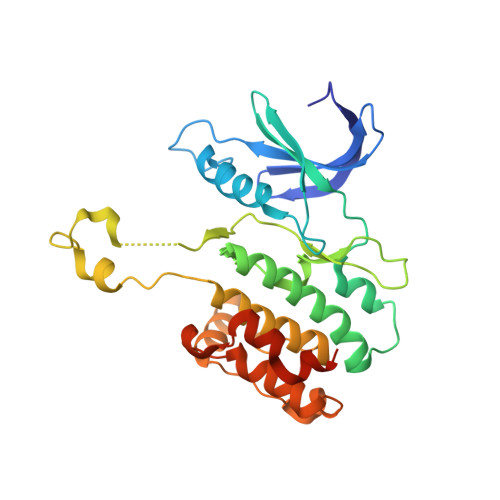
De Novo Fragment Design for Drug Discovery and Chemical Biology.
Rodrigues, T., Reker, D., Welin, M., Caldera, M., Brunner, C., Gabernet, G., Schneider, P., Walse, B., Schneider, G.(2015) Angew Chem Int Ed Engl 54: 15079
- PubMed: 26486226
- DOI: https://doi.org/10.1002/anie.201508055
- Primary Citation of Related Structures:
5A6N, 5A6O - PubMed Abstract:
Automated molecular de novo design led to the discovery of an innovative inhibitor of death-associated protein kinase 3 (DAPK3). An unprecedented crystal structure of the inactive DAPK3 homodimer shows the fragment-like hit bound to the ATP pocket. Target prediction software based on machine learning models correctly identified additional macromolecular targets of the computationally designed compound and the structurally related marketed drug azosemide. The study validates computational de novo design as a prime method for generating chemical probes and starting points for drug discovery.
- Swiss Federal Institute of Technology (ETH Zürich), Vladimir-Prelog-Weg 4, 8093 Zürich (Switzerland).
Organizational Affiliation: