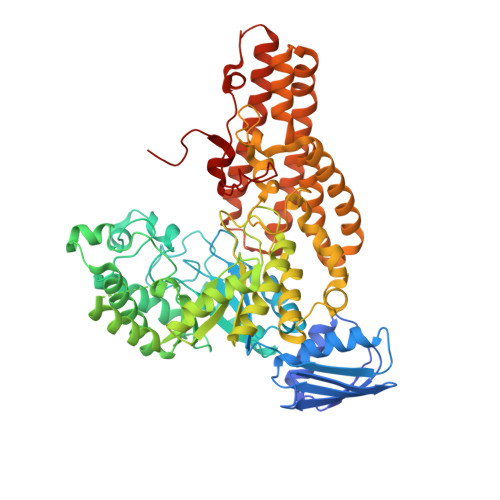
A Second beta-Hexosaminidase Encoded in the Streptococcus pneumoniae Genome Provides an Expanded Biochemical Ability to Degrade Host Glycans.
Robb, M., Robb, C.S., Higgins, M.A., Hobbs, J.K., Paton, J.C., Boraston, A.B.(2015) J Biological Chem 290: 30888-30900
- PubMed: 26491009
- DOI: https://doi.org/10.1074/jbc.M115.688630
- Primary Citation of Related Structures:
5A69, 5A6A, 5A6B, 5A6J, 5A6K, 5AC4, 5AC5 - PubMed Abstract:
An important facet of the interaction between the pathogen Streptococcus pneumoniae (pneumococcus) and its human host is the ability of this bacterium to process host glycans. To achieve cleavage of the glycosidic bonds in host glycans, S. pneumoniae deploys a wide array of glycoside hydrolases. Here, we identify and characterize a new family 20 glycoside hydrolase, GH20C, from S. pneumoniae. Recombinant GH20C possessed the ability to hydrolyze the β-linkages joining either N-acetylglucosamine or N-acetylgalactosamine to a wide variety of aglycon residues, thus revealing this enzyme to be a generalist N-acetylhexosaminidase in vitro. X-ray crystal structures were determined for GH20C in a ligand-free form, in complex with the N-acetylglucosamine and N-acetylgalactosamine products of catalysis and in complex with both gluco- and galacto-configured inhibitors O-(2-acetamido-2-deoxy-D-glucopyranosylidene)amino N-phenyl carbamate (PUGNAc), O-(2-acetamido-2-deoxy-D-galactopyranosylidene)amino N-phenyl carbamate (GalPUGNAc), N-acetyl-D-glucosamine-thiazoline (NGT), and N-acetyl-D-galactosamine-thiazoline (GalNGT) at resolutions from 1.84 to 2.7 Å. These structures showed N-acetylglucosamine and N-acetylgalactosamine to be recognized via identical sets of molecular interactions. Although the same sets of interaction were maintained with the gluco- and galacto-configured inhibitors, the inhibition constants suggested preferred recognition of the axial O4 when an aglycon moiety was present (Ki for PUGNAc > GalPUGNAc) but preferred recognition of an equatorial O4 when the aglycon was absent (Ki for GalNGT > NGT). Overall, this study reveals GH20C to be another tool that is unique in the arsenal of S. pneumoniae and that it may implement the effort of the bacterium to utilize and/or destroy the wide array of host glycans that it may encounter.
- From the Department of Biochemistry and Microbiology, University of Victoria, Victoria, British Columbia, Canada V8W 3P6 and.
Organizational Affiliation: