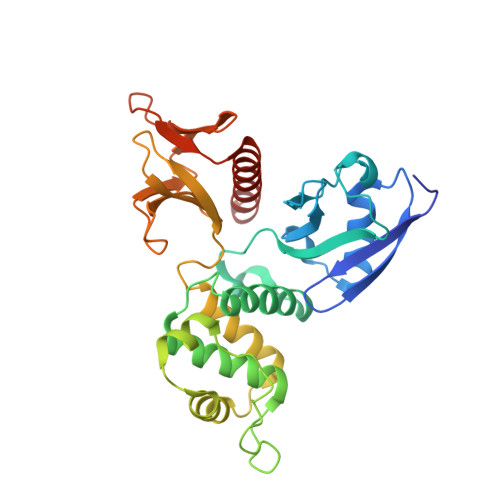
Structural Basis for the Phosphorylation-regulated Interaction between the Cytoplasmic Tail of Cell Polarity Protein Crumbs and the Actin-binding Protein Moesin
Wei, Z., Li, Y., Ye, F., Zhang, M.(2015) J Biological Chem 290: 11384-11392
- PubMed: 25792740
- DOI: https://doi.org/10.1074/jbc.M115.643791
- Primary Citation of Related Structures:
4YL8 - PubMed Abstract:
The type I transmembrane protein crumbs (Crb) plays critical roles in the establishment and maintenance of cell polarities in diverse tissues. As such, mutations of Crb can cause different forms of cancers. The cell intrinsic role of Crb in cell polarity is governed by its conserved, 37-residue cytoplasmic tail (Crb-CT) via binding to moesin and protein associated with Lin7-1 (PALS1). However, the detailed mechanism governing the Crb·moesin interaction and the balance of Crb in binding to moesin and PALS1 are not well understood. Here we report the 1.5 Å resolution crystal structure of the moesin protein 4.1/ezrin/radixin/moesin (FERM)·Crb-CT complex, revealing that both the canonical FERM binding motif and the postsynaptic density protein-95/Disc large-1/Zonula occludens-1 (PDZ) binding motif of Crb contribute to the Crb·moesin interaction. We further demonstrate that phosphorylation of Crb-CT by atypical protein kinase C (aPKC) disrupts the Crb·moesin association but has no impact on the Crb·PALS1 interaction. The above results indicate that, upon the establishment of the apical-basal polarity in epithelia, apical-localized aPKC can actively prevent the Crb·moesin complex formation and thereby shift Crb to form complex with PALS1 at apical junctions. Therefore, Crb may serve as an aPKC-mediated sensor in coordinating contact-dependent cell growth inhibition in epithelial tissues.
- From the Division of Life Science, State Key Laboratory of Molecular Neuroscience, Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China, Center of Systems Biology and Human Health, School of Science and Institute for Advanced Study, Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China, and Department of Biology, South University of Science and Technology of China, Shenzhen 518055, China.
Organizational Affiliation: