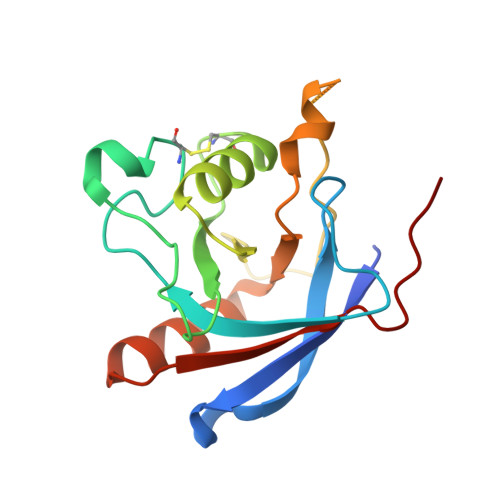
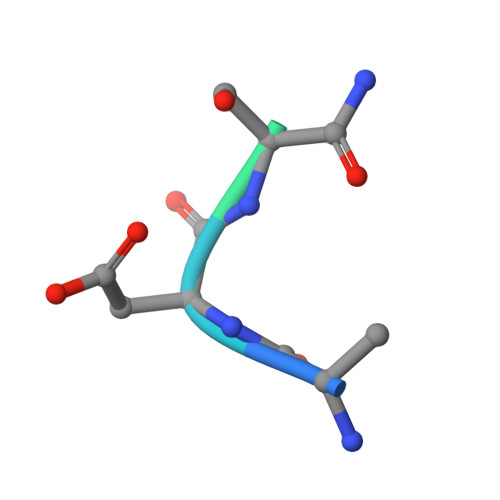
How vacuolar sorting receptor proteins interact with their cargo proteins: crystal structures of apo and cargo-bound forms of the protease-associated domain from an Arabidopsis vacuolar sorting receptor.
Luo, F., Fong, Y.H., Zeng, Y., Shen, J., Jiang, L., Wong, K.B.(2014) Plant Cell 26: 3693-3708
- PubMed: 25271241
- DOI: https://doi.org/10.1105/tpc.114.129940
- Primary Citation of Related Structures:
4TJV, 4TJX - PubMed Abstract:
In plant cells, soluble proteins are directed to vacuoles because they contain vacuolar sorting determinants (VSDs) that are recognized by vacuolar sorting receptors (VSR). To understand how a VSR recognizes its cargo, we present the crystal structures of the protease-associated domain of VSR isoform 1 from Arabidopsis thaliana (VSR1PA) alone and complexed with a cognate peptide containing the barley (Hordeum vulgare) aleurain VSD sequence of 1ADSNPIRPVT10. The crystal structures show that VSR1PA binds the sequence, Ala-Asp-Ser, preceding the NPIR motif. A conserved cargo binding loop, with a consensus sequence of 95RGxCxF100, forms a cradle that accommodates the cargo-peptide. In particular, Arg-95 forms a hydrogen bond to the Ser-3 position of the VSD, and the essential role of Arg-95 and Ser-3 in receptor-cargo interaction was supported by a mutagenesis study. Cargo binding induces conformational changes that are propagated from the cargo binding loop to the C terminus via conserved residues in switch I-IV regions. The resulting 180° swivel motion of the C-terminal tail is stabilized by a hydrogen bond between Glu-24 and His-181. A mutagenesis study showed that these two residues are essential for cargo interaction and trafficking. Based on our structural and functional studies, we present a model of how VSRs recognize their cargos.
- Centre for Protein Science and Crystallography, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong, China Centre for Cell and Developmental Biology and State Key Laboratory of Agrobiotechnology, School of Life Sciences, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong, China.
Organizational Affiliation: