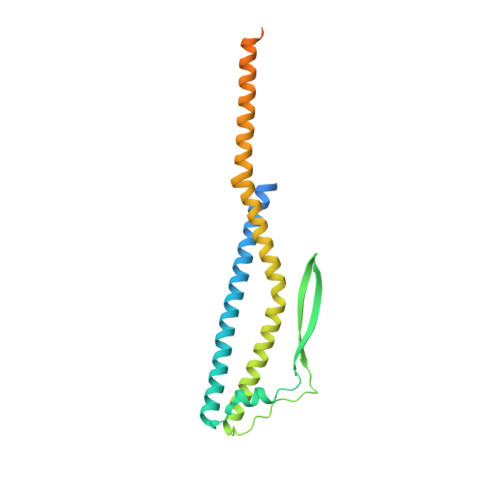
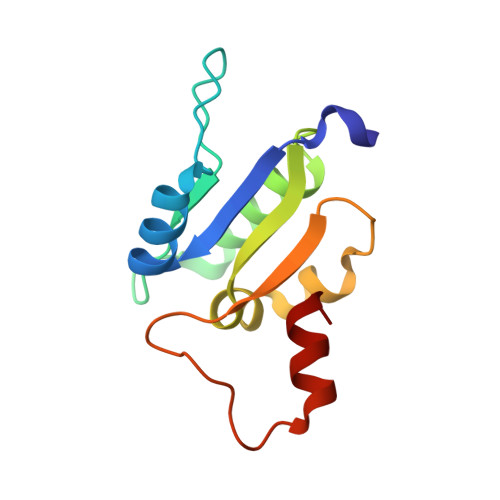
Crystal Structure of Subunits D and F in Complex Gives Insight into Energy Transmission of the Eukaryotic V-ATPase from Saccharomyces cerevisiae.
Balakrishna, A.M., Basak, S., Manimekalai, M.S., Gruber, G.(2015) J Biological Chem 290: 3183-3196
- PubMed: 25505269
- DOI: https://doi.org/10.1074/jbc.M114.622688
- Primary Citation of Related Structures:
4RND - PubMed Abstract:
Eukaryotic V1VO-ATPases hydrolyze ATP in the V1 domain coupled to ion pumping in VO. A unique mode of regulation of V-ATPases is the reversible disassembly of V1 and VO, which reduces ATPase activity and causes silencing of ion conduction. The subunits D and F are proposed to be key in these enzymatic processes. Here, we describe the structures of two conformations of the subunit DF assembly of Saccharomyces cerevisiae (ScDF) V-ATPase at 3.1 Å resolution. Subunit D (ScD) consists of a long pair of α-helices connected by a short helix ((79)IGYQVQE(85)) as well as a β-hairpin region, which is flanked by two flexible loops. The long pair of helices is composed of the N-terminal α-helix and the C-terminal helix, showing structural alterations in the two ScDF structures. The entire subunit F (ScF) consists of an N-terminal domain of four β-strands (β1-β4) connected by four α-helices (α1-α4). α1 and β2 are connected via the loop (26)GQITPETQEK(35), which is unique in eukaryotic V-ATPases. Adjacent to the N-terminal domain is a flexible loop, followed by a C-terminal α-helix (α5). A perpendicular and extended conformation of helix α5 was observed in the two crystal structures and in solution x-ray scattering experiments, respectively. Fitted into the nucleotide-bound A3B3 structure of the related A-ATP synthase from Enterococcus hirae, the arrangements of the ScDF molecules reflect their central function in ATPase-coupled ion conduction. Furthermore, the flexibility of the terminal helices of both subunits as well as the loop (26)GQITPETQEK(35) provides information about the regulatory step of reversible V1VO disassembly.
- From the School of Biological Sciences, Nanyang Technological University, 60 Nanyang Drive, Singapore 637551, Republic of Singapore.
Organizational Affiliation: