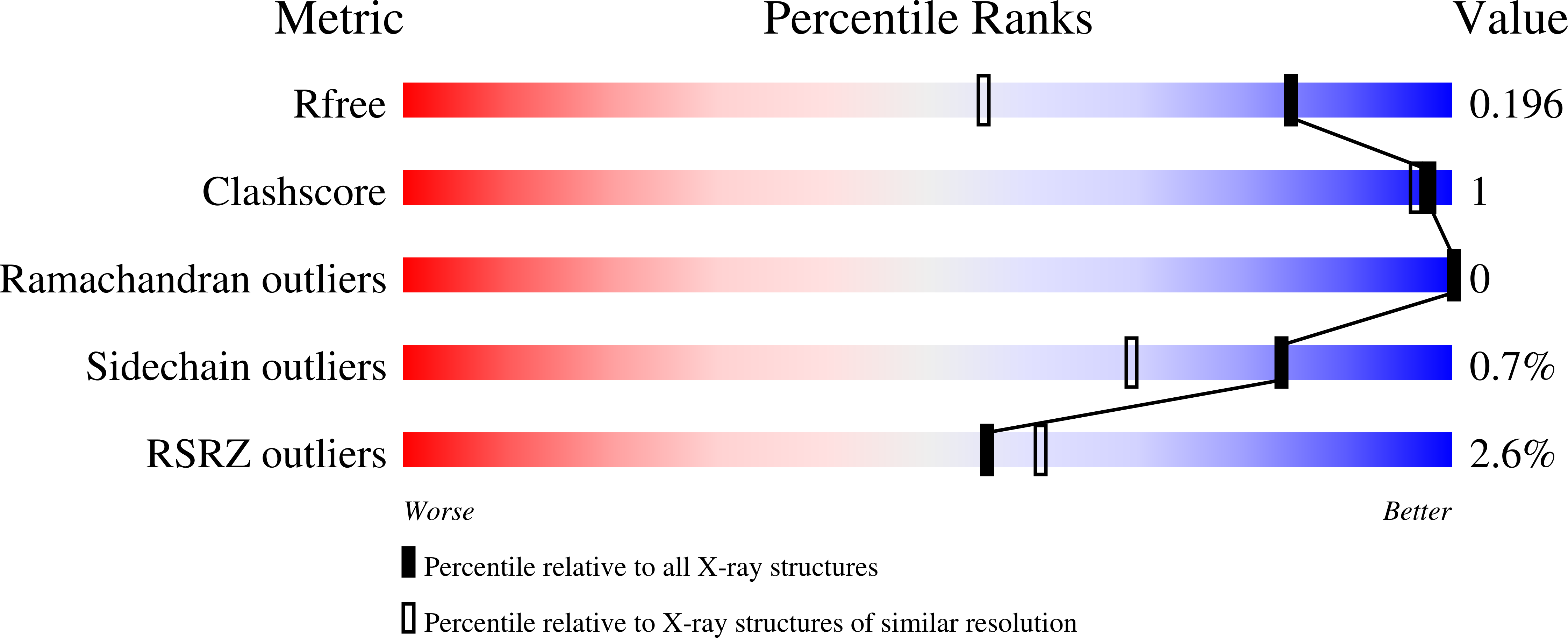
Crystal structures and inhibitor binding properties of plant class V chitinases: the cycad enzyme exhibits unique structural and functional features.
Umemoto, N., Kanda, Y., Ohnuma, T., Osawa, T., Numata, T., Sakuda, S., Taira, T., Fukamizo, T.(2015) Plant J 82: 54-66
- PubMed: 25652217
- DOI: https://doi.org/10.1111/tpj.12785
- Primary Citation of Related Structures:
4R5E - PubMed Abstract:
A class V (glycoside hydrolase family 18) chitinase from the cycad Cycas revoluta (CrChiA) is a plant chitinase that has been reported to possess efficient transglycosylation (TG) activity. We solved the crystal structure of CrChiA, and compared it with those of class V chitinases from Nicotiana tabacum (NtChiV) and Arabidopsis thaliana (AtChiC), which do not efficiently catalyze the TG reaction. All three chitinases had a similar (α/β)8 barrel fold with an (α + β) insertion domain. In the acceptor binding site (+1, +2 and +3) of CrChiA, the Trp168 side chain was found to stack face-to-face with the +3 sugar. However, this interaction was not found in the identical regions of NtChiV and AtChiC. In the DxDxE motif, which is essential for catalysis, the carboxyl group of the middle Asp (Asp117) was always oriented toward the catalytic acid Glu119 in CrChiA, whereas the corresponding Asp in NtChiV and AtChiC was oriented toward the first Asp. These structural features of CrChiA appear to be responsible for the efficient TG activity. When binding of the inhibitor allosamidin was evaluated using isothermal titration calorimetry, the changes in binding free energy of the three chitinases were found to be similar to each other, i.e. between -9.5 and -9.8 kcal mol(-1) . However, solvation and conformational entropy changes in CrChiA were markedly different from those in NtChiV and AtChiC, but similar to those of chitinase A from Serratia marcescens (SmChiA), which also exhibits significant TG activity. These results provide insight into the molecular mechanism underlying the TG reaction and the molecular evolution from bacterial chitinases to plant class V chitinases.
Organizational Affiliation:
Department of Advanced Bioscience, Kinki University, 3327-204 Nakamachi, Nara, 631-8505, Japan.