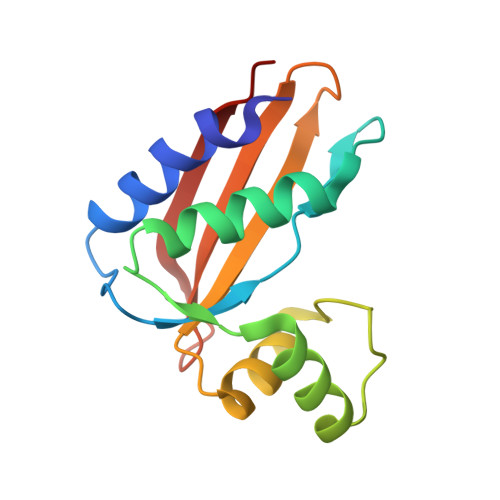
Structural Insights into the Lipoprotein Outer Membrane Regulator of Penicillin-binding Protein 1B.
King, D.T., Lameignere, E., Strynadka, N.C.(2014) J Biological Chem 289: 19245-19253
- PubMed: 24808177
- DOI: https://doi.org/10.1074/jbc.M114.565879
- Primary Citation of Related Structures:
4Q6L, 4Q6V, 4Q6Z - PubMed Abstract:
In bacteria, the synthesis of the protective peptidoglycan sacculus is a dynamic process that is tightly regulated at multiple levels. Recently, the lipoprotein co-factor LpoB has been found essential for the in vivo function of the major peptidoglycan synthase PBP1b in Enterobacteriaceae. Here, we reveal the crystal structures of Salmonella enterica and Escherichia coli LpoB. The LpoB protein can be modeled as a ball and tether, consisting of a disordered N-terminal region followed by a compact globular C-terminal domain. Taken together, our structural data allow us to propose new insights into LpoB-mediated regulation of peptidoglycan synthesis.
- From the Department of Biochemistry and Molecular Biology and Centre for Blood Research, University of British Columbia, Vancouver, British Columbia V6T 1Z3, Canada.
Organizational Affiliation: